[Solved] Draw lewis structure for Sulfite ion SO 3 2 Course Hero
Lewis structures are preferable when adjacent formal charges are zero or of the opposite sign. When we must choose among several Lewis structures with similar distributions of formal charges, the structure with the negative formal charges on the more electronegative atoms is preferable.. In the sulfite ion,.
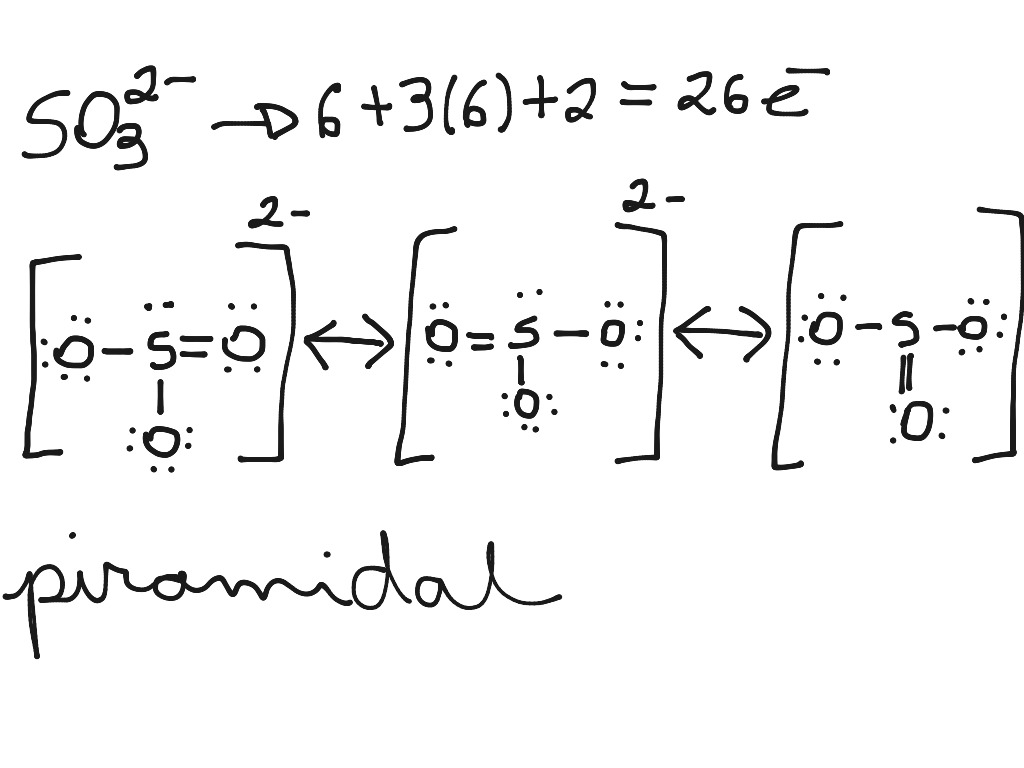
Sulfite ion Science, Chemistry, Chemical Bonds ShowMe
How to Draw the Lewis Dot Structure for S 2- (Sulfide ion) Wayne Breslyn 724K subscribers Subscribe Subscribed 461 103K views 5 years ago A step-by-step explanation of how to draw the S2-.
[Solved] Draw lewis structure for Sulfite ion SO 3 2 Course Hero
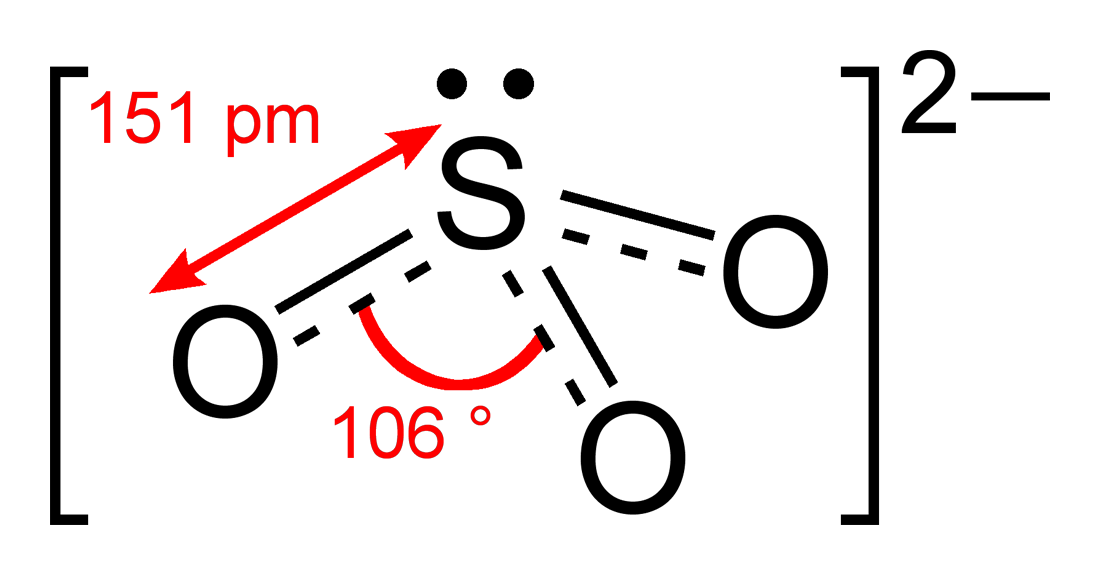
The structure of the sulfite anion Sulfite is a ligand in coordination chemistry. The structure of Co ( ethylenediamine) 2 (SO 3 )N 3. [4] The structure of the sulfite anion can be described with three equivalent resonance structures.

Ionic Bonding Elements are the simplest substances There
the greater number of valence electrons found in heteronuclear molecules. Answer. Exercise 8.9.2 8.9. 2. In molecules, as bond order increases, both bond length and bond energy increase. both bond length and bond energy decrease. bond length increases and bond energy is unchanged. bond length is unchanged and bond energy increases.

SO3 2 Lewis Structure (Sulfite Ion) YouTube
Video 8.5.2: Please look at Handout 1: Lewis dot structure technique while watching this video. Note steps 1 and 2 are switched but all the rest are the same. Question: What is the formal charge of each atom in the Lewis dot structure of sulfite? Answer The sulfur has a [+1] formal charge as it donates 6 electrons to the structure, but has 5.

SO3 2 Lewis Structure How to Draw the Lewis Structure for SO3 2
A step-by-step explanation of how to draw the SO3 2- Lewis Structure (Sulfite Ion). For the SO3 2- Lewis structure the total number of valence electrons.

So3 схема химической связи 82 фото
The hydroxide ion, though, contains an octet of electrons, one more than the neutral molecule. The hydroxide ion must thus carry a single negative charge. In order to draw the Lewis structure for a given ion, we must first determine how many valence electrons are involved. Suppose the structure of H 3 O + is required. The total number of.
Lewis Structure Sulfur Diagram Bohr Model Electron Hydrogen Sulfide
It is useful to see what can be conveyed via 3D models, molecular geometry for instance, and what is conveyed by Lewis structure models, an example being the assignment of electrons around atoms and assignment of electrons for bonding. An important clarification, is that the twirlymols display connections, not bonds, so double and triple bonds.

Molécula Del Anión Del Sulfito Sulfitos De Los Sulfitos Que Contienen
Lewis Dot Structure of SO3 2- (Sulfite Ion) kentchemistry.com 25.1K subscribers Subscribe Subscribed 196K views 12 years ago Every Video I quickly take you through how to draw the Lewis.
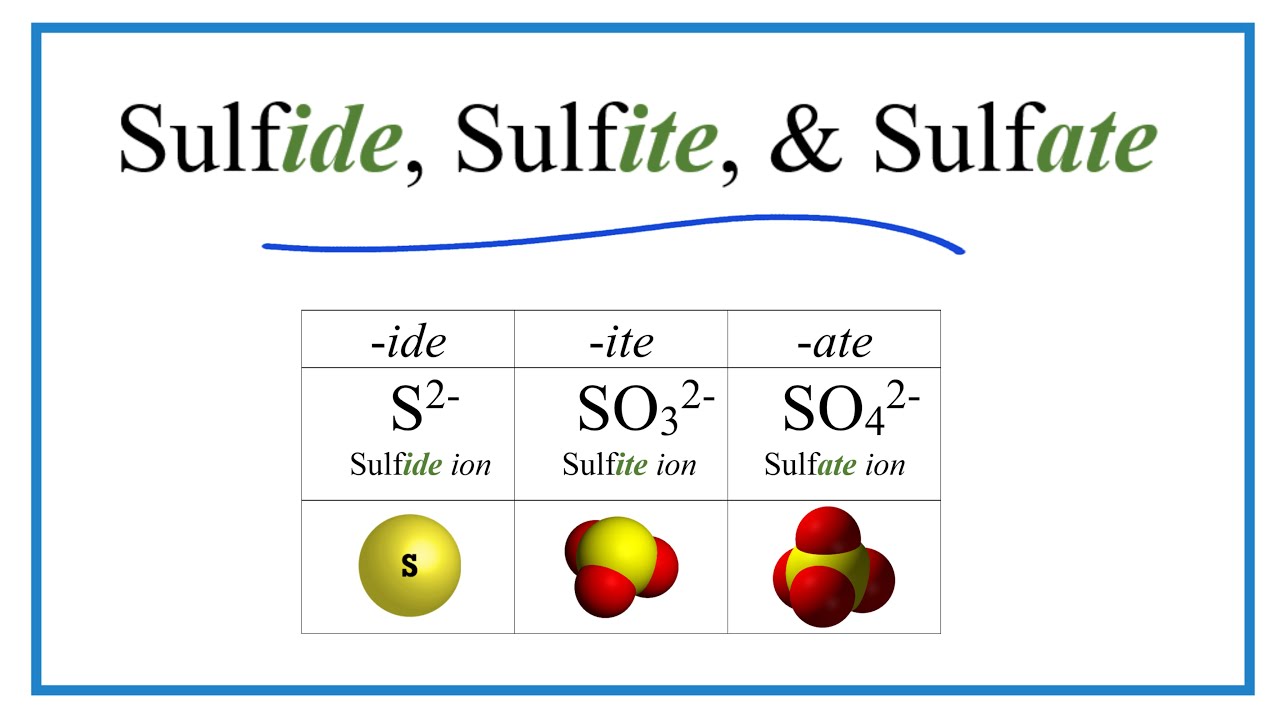
Sulfide, Sulfite, Sulfate Ions (Difference And Formulas) vlr.eng.br
Sulfite ion is a weak base, but does undergo some hydrolysis to produce basic solutions. In acidic solution, the equilibria are shifted to form sulfurous acid, resulting in the evolution of SO2 gas. Sulfur dioxide is a colorless gas with a characteristic choking odor. SO2−3 (aq) +H2O(l) ↽−−⇀ HSO−3 (aq) +OH−(aq) SO 3 2 − ( aq.

FileSulfateion2Ddimensions.png Wikimedia Commons
We are going to determine the Lewis structure for SO3 2- minus ion, also known as sulfide ion. The -2 charge you see is because it accepts two additional electrons, giving the ion a negative charge. Step-by-Step Guide to Drawing the SO3 2- Lewis Structure 1. Count Valence Electrons
Sulfite
The sulfite ion comprises one Sulfur Atom and three Oxygen atoms. The ion has a negative.more.more Hello Guys!The sulfite ion comprises one Sulfur Atom and three Oxygen atoms. The.

So3 Lewis Structure With Formal Charges
Lewis structure of sulfite ion is drawn in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure. Resonance structures of SO 32- are drawn after drawing the lewis structure. Sulfite ion | sulphite ion | SO 32- Sulfite ion is one of the oxyanion of sulfur. Sulfur is at +4 oxidation state in SO 32-.

Sulfur S (Element 16) of Periodic Table Elements FlashCards
Lewis Dot of the Sulfite Ion SO 32- Back 70 More Lewis Dot Structures S does not follow the octet rule. It will hold more than 8 electrons. Sulfur having valence electrons in the 3rd energy level, will also have access to the 3d sublevel, thus allowing for more than 8 electrons.
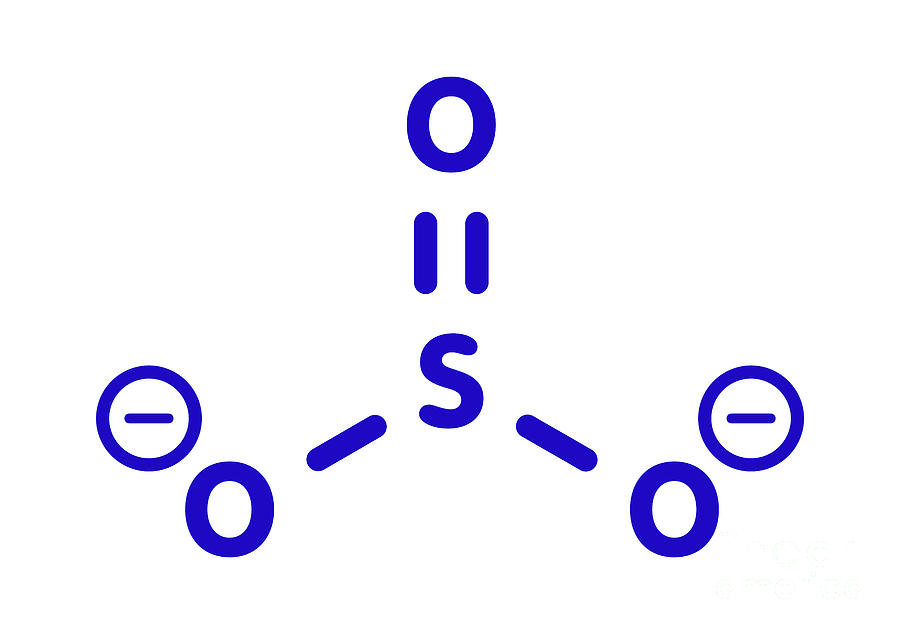
Sulfite Anion Chemical Structure Photograph by Molekuul/science Photo
Lewis structures are another way to represent molecules. Lewis Structures were introduced by Gilbert N. Lewis in 1916.. If the ion had been SO 3 2-(sulfite) instead of sulfate, then the structure would have had a lone pair of electrons on the central atom: The structure of PCl 5 is a good example of a molecule that exceeds the octet:

Sodium sulfite YouTube
In this video, you will learn how to draw the lewis structure for chemicals based on total valence electrons, the octet rule, duet rule, and also you will le.