
14 Stoichiometry Worksheet 2 Answer Key /
Name MURDER MYSTERY: Stoichiometry Edition You are the lead investigator on the case of a murdered athlete.. KEY Stoichiometry Murder Mystery Project.pdf. Solutions Available. Inwood School. ENGLISH 08.. independence from suppliers and customers Answer False LG 4LL 2 Page 193.
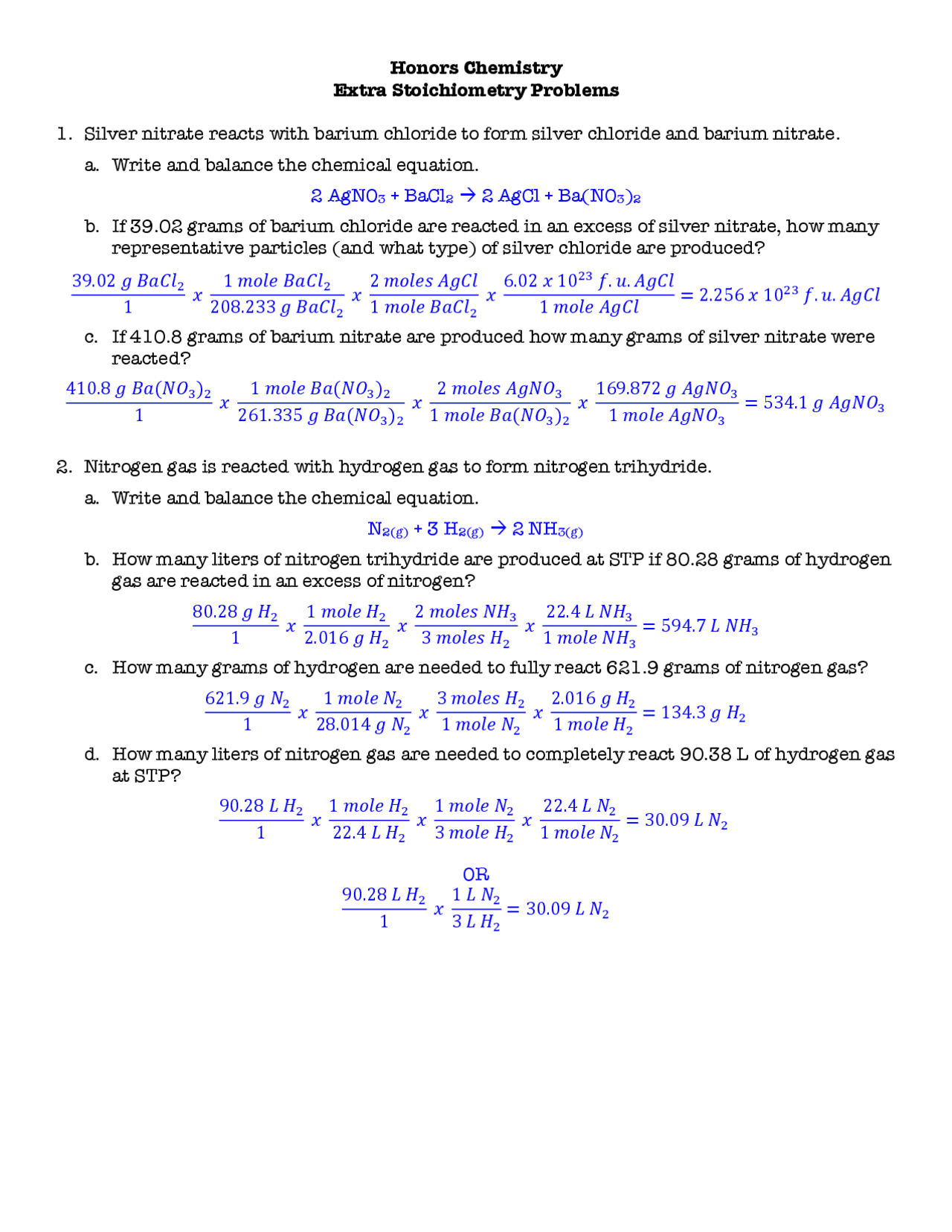
Stoichiometry Problems Worksheet Answers
The knife had 10 grams of rust. The minimum mass of a knife should be 60 grams if it is used in a murder. Mass of rust is given, so first, calculate the moles of Fe(OH)3. Compare the moles of Fe(OH)3 with moles of Fe and find the mole ratio between Fe(OH)3 and Fe using stoichiometry of the reaction.

Pin on Stoichiometry and The Mole
Help your students practice gram-to-gram stoichiometry problems with this fun, creative project! This assignment includes a murder mystery scenario that involves four potential culprits. Students will use stoichiometry to "investigate" the scene of the crime and determine which suspect is guilty. Afterwards, students can use their stoichiometry.

Answer Sheet Template
Our development team has been informed of the issue. Help your students practice gram-to-gram stoichiometry problems with this fun, creative project! This assignment includes a murder mystery scenario that involves four potential culprits. Students will use stoichiometry to "investigate" the scene of the crime and determine which suspect is.

Lesson Inference MysteryThe Murder at Blue Mountain Lodge YouTube
Activities. trueTV has a multitude of resources. You can begin by taping an episode or two of " Forensic Files " to show how actual forensic scientists solve cases and have students fill out this worksheet ( doc) as they watch the program. Check out their forensic lab. After reading " Crime Scene Processing " on the Forensic Science Web.

Stoichiometry Project Crossword WordMint
Multiplying the number of moles of H A 2 SO A 4 by this factor gives us the number of moles of NaOH needed: 3.16 × 10 − 2 mol H 2 SO 4 × 2 mol NaOH 1 mol H 2 SO 4 = 6.32 × 10 − 2 mol NaOH. Notice how we wrote the mole ratio so that the moles of H A 2 SO A 4 cancel out, resulting in moles of NaOH as the final units.

Stoichiometry General Knowledge Multiple Choice Questions(MCQs) with
Name: Shreyaan Jain Period: 3 Stoichiometry Clue - Who did it? Use the clues to determine the identity of the murderer, the weapon, and the location. Write and balance all chemical equations. Show all work: Even Nobel Prize-winning scientists must show their work. Write UNITS and FORMULAS next to each number as you solve the problems. If you end up getting problems wrong, check that you have.
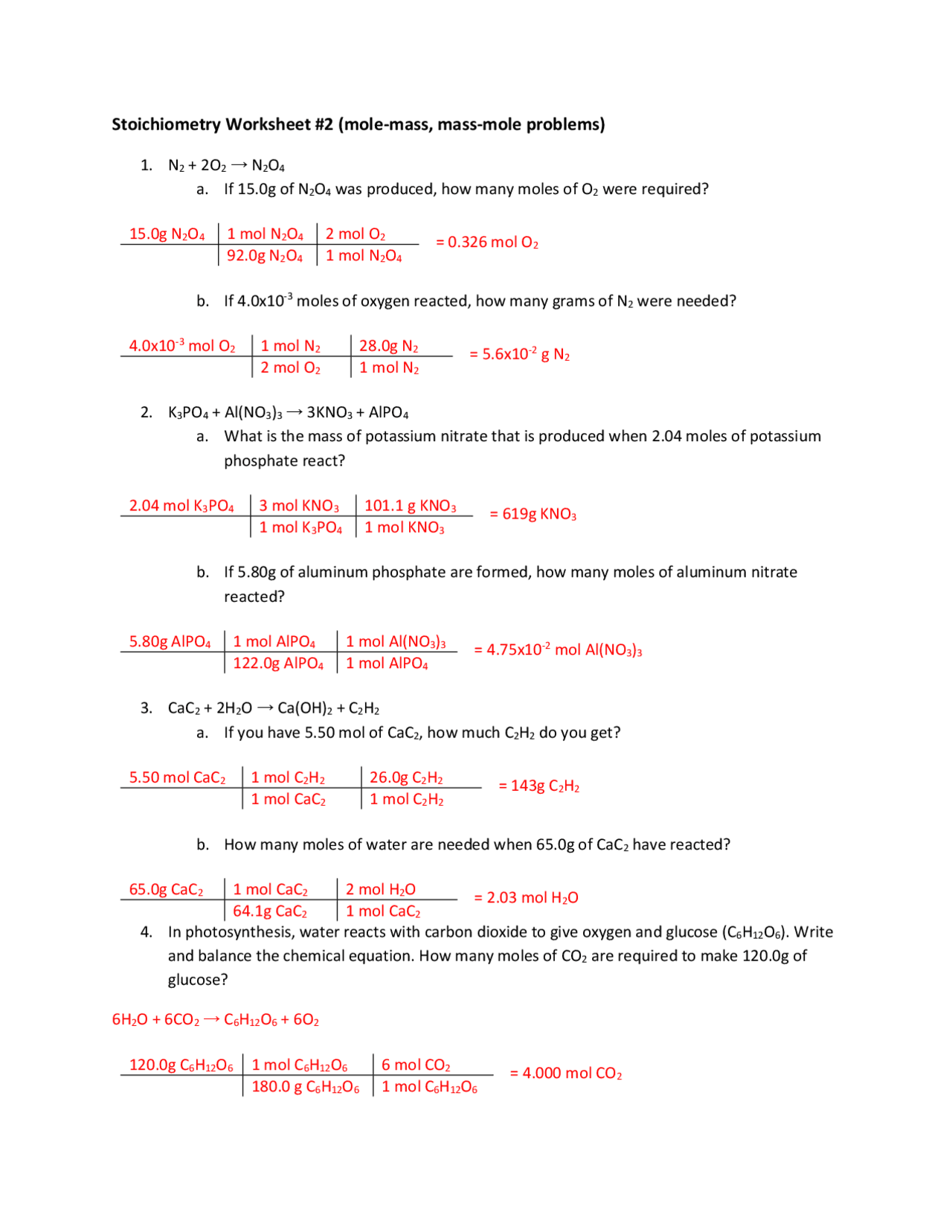
Stoichiometry Worksheet Answer Key
Host virtual events and webinars to increase engagement and generate leads.
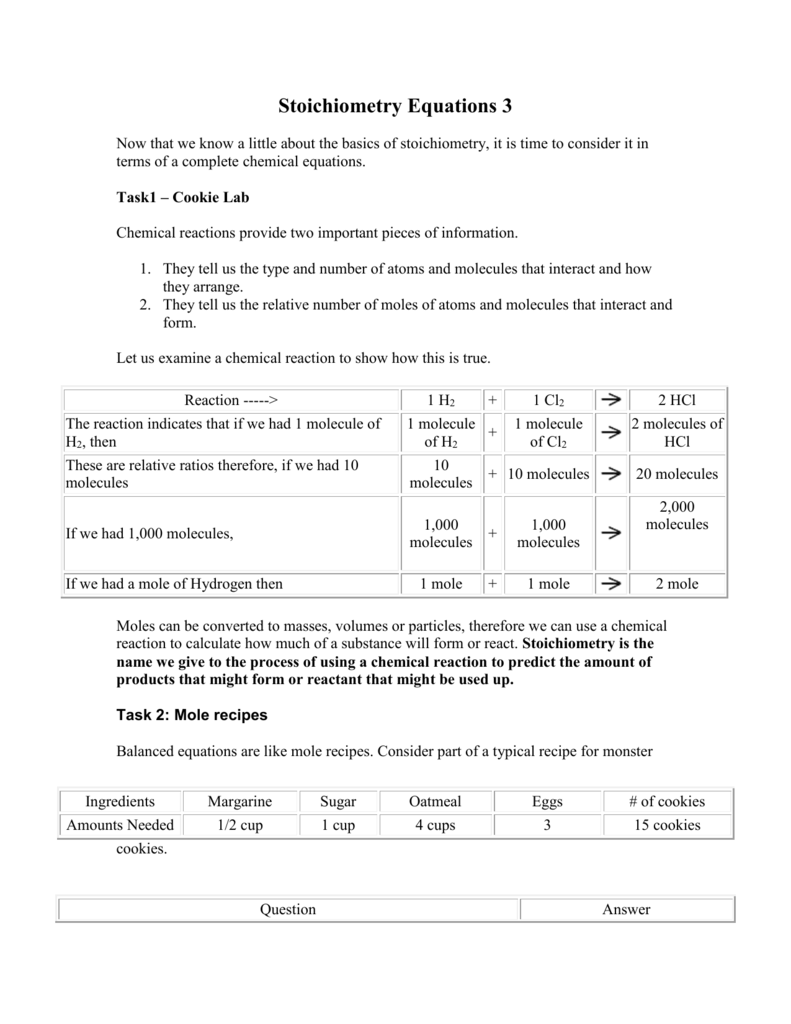
Stoichiometry Equations 3
Names: Karla Portilla & Gabriel Serrano Block: 5 MURDER MYSTERY: Stoichiometry Edition You are the lead investigator on the case of a murdered athlete. You must find out how the victim was killed and who killed him, using your knowledge of stoichiometry. Read the instructions, find the evidence, and solve the case. Victim information: Case # 153 Name: Jasper Chan Age: 24 Sex: M Height: 5'10.

Stoichiometry Problem Set ANSWERS.pdf
Question: Stoichiometry Murder Mystery The wealthy businessman Bruce Rockefeller walked into his home last night to find his butler dead on the floor. Before Bruce could check the body, police stormed through the door and tackled him to the ground. Bruce was arrested immediately, but he insisted that he was innocent The police also arrested.

Stoichiometry+Problems
Suspect #3: Grizzly Greg The third suspect arrested outside of the house was a large man named Greg. He was arrested in previous years for setting his pets (a dog and three cats) on fire in his backyard. Police think that he might be the murderer. When police investigated the house, they found a suspicious chemical called methanol (CH 4 O). Methanol is a flammable liquid often used to set.

Stoichiometry Tutorial Pathways to Chemistry
Resource Topic: Stoichiometry . The Mole, Molarity, and Density. Autograded Virtual Labs; Metals Density Problem Autograded Virtual Lab. In this activity, students use the virtual lab to identify 3 unknown metals by measuring their density and comparing their measurements to the densities of known metals. In this randomized version, each student…

Chemistry Advanced Stoichiometry Mystery (1 Teacher License)
Chemistry. 10 Grade. Waylon Whitney. Copy and edit PRO. Best for asynchronous learning and homework Assign in student-paced mode. Best for live in-class or video conferencing lessons Start teacher-led lesson. 4 slides. Show answers. Preview as student.

How To Solve Stoichiometry Problems YouTube
A crime has been committed and we have to use our knowledge of solution chemistry to solve the crime. Miss Scarlet was found dead on the floor of the ballroom. Beside her body, police found a clear liquid, the murder weapon. Detective found out that it was one of two chemicals. They knew that Miss Scarlet has a violent allergic reaction to.

Stoichiometry Review Part 1 Chemistry Quizizz
Description. Great worksheet of questions to introduce mole-to-mole stoichiometry. This free product contains a set of practice problems that can be done together in class. The questions also make perfect homework problems for students learning about mole-to-mole stoichiometry. For a more expanded, in-depth version of this product, see the.

Stoichiometry Problems Part 2 YouTube
How many moles of water are produced when 57 moles of nitrogen are made? 3. Calculate the mass of aluminum oxide produced when 3.75 moles of aluminum burn in oxygen. Answers: 1A. 30 mol Ag 1B. 30 mol AgNO3. 1C. 20 mol H2O 1D. 10 mol NO. 2A. 38 mol N2H4 2B. 19 mol N2O4. 2C. 76 mol H2O.