
XeF4 Lewis structure, Molecular geometry, Bond angle, Shape
Science Chemistry Chemistry questions and answers Write the Lewis structure for XeF4. Draw the molecule by placing atoms on the canvas and connecting them with bonds. Include all lone pairs of electrons. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer

Hello Guys! Today we are going to look at the Lewis Structure of XeF4
XeF4 is the chemical formula of the compound Xenon Tetrafluoride. This chemical compound is formed when xenon reacts with fluorine. Its chemical equation could simply be written as : Xe + 2F2 ——> XeF4 In this process, elemental fluorine supposedly oxidizes xenon, under some specific conditions of temperature and pressure.
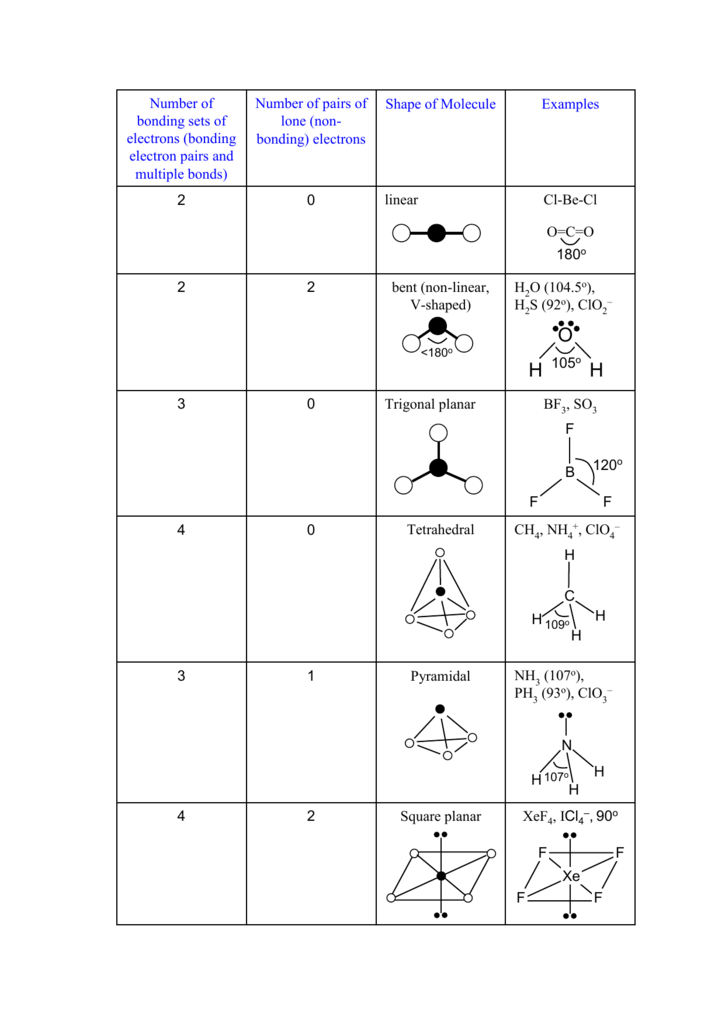
XeF4, ICl4 , 90o Square planar 2 4 NH (107o), PH (93o), ClO
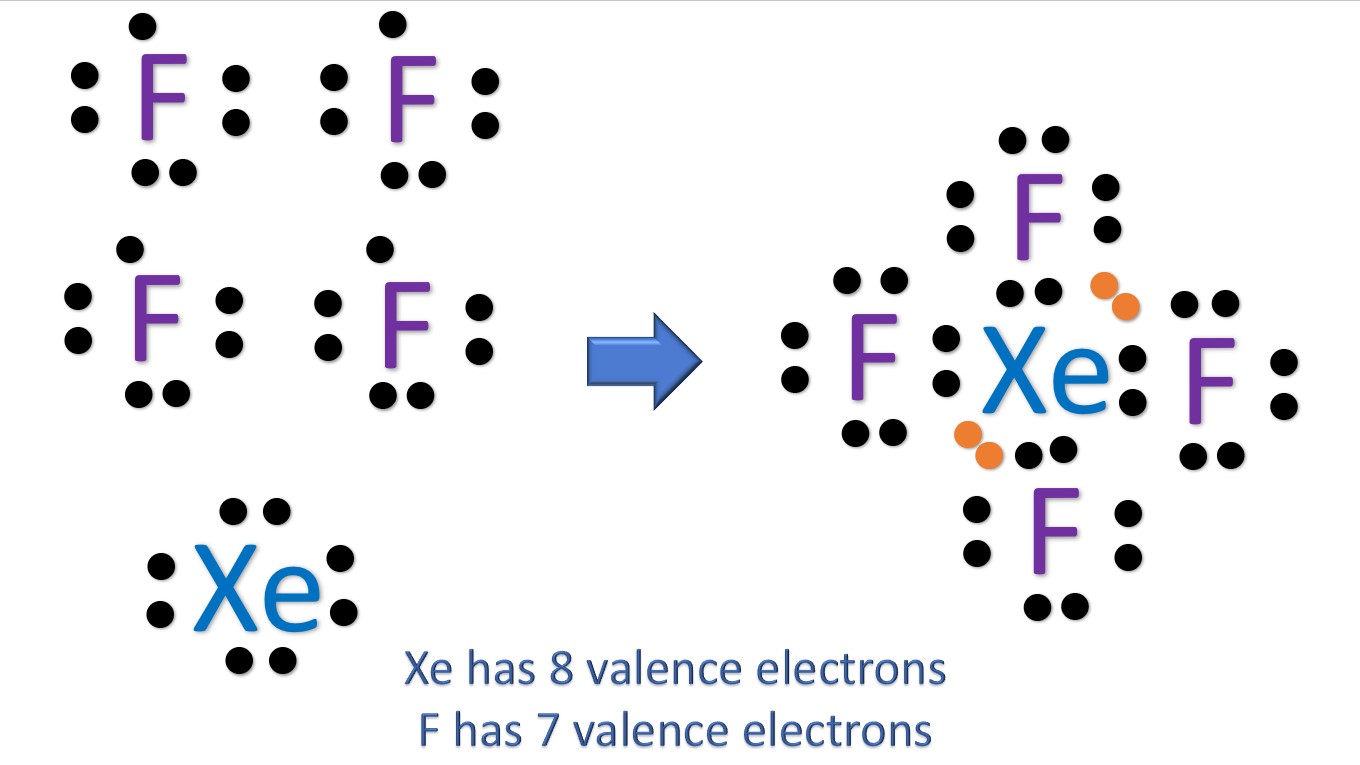
For the XeF4 Lewis structure we first count the valence electrons for the XeF4 molecule using the periodic table. Once we know how many valence electrons there are in XeF4 we can distribute them around the central atom and attempt to fill the outer shells of each atom. The Lewis structure for XeF4 has a total of 36 valence electrons.

Leave a Comment Cancel Reply
For making the Lewis structure, we need to know the valence electrons of XeF4 to make its structure and know the placement of atoms in the molecule. Contents XeF4 Valence electrons XeF4 Lewis Structure XeF4 Hybridization XeF4 Molecular Geometry XeF4 Bond angles XeF4 Polarity - Is XeF4 Polar or Nonpolar? XeF4 Valence electrons

Solved The Lewis structure for XeF4 is Xě Which of
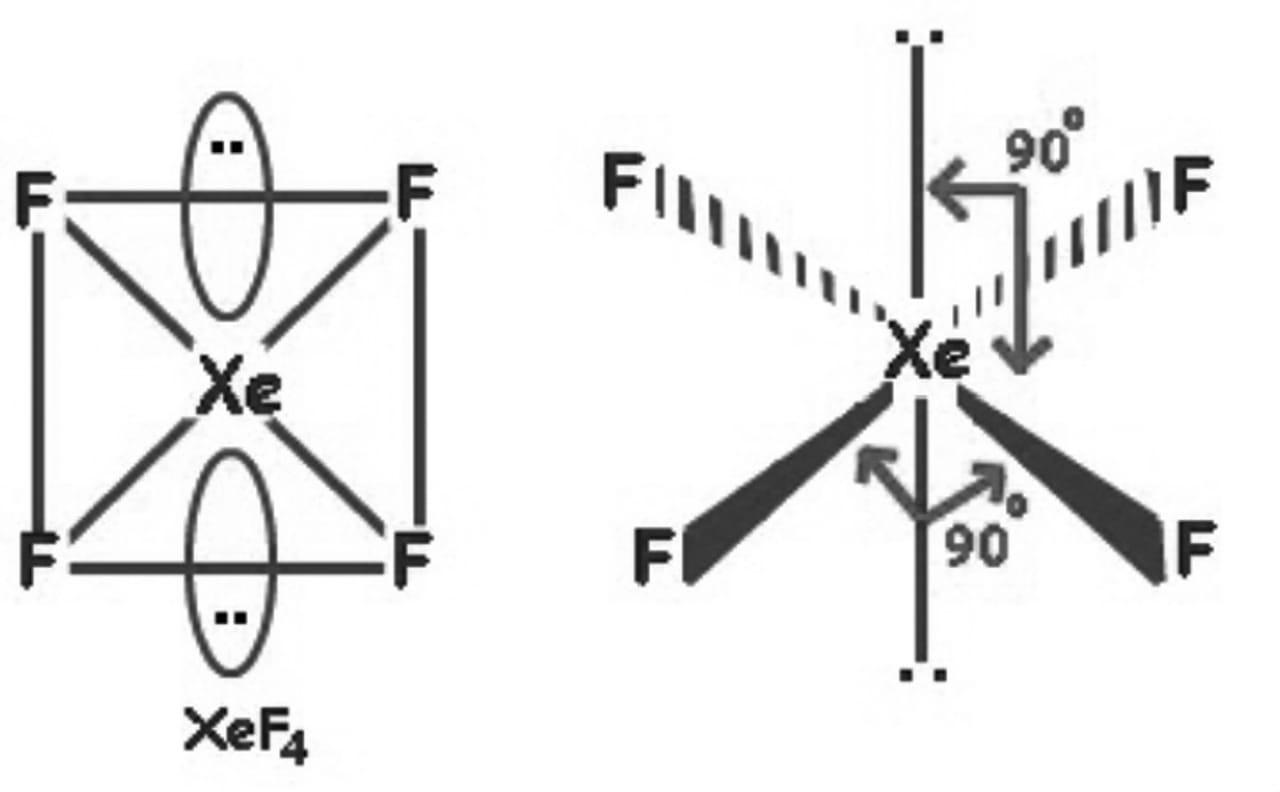
XeF4 lewis structure has Xenon atom (Xe) at the center which is surrounded by four Fluorine atoms (F). There are 4 single bonds between the Xenon atom (Xe) and each Fluorine atom (F). There are 2 lone pairs on the Xenon atom (Xe) and 3 lone pairs on all the four Fluorine atoms (F).
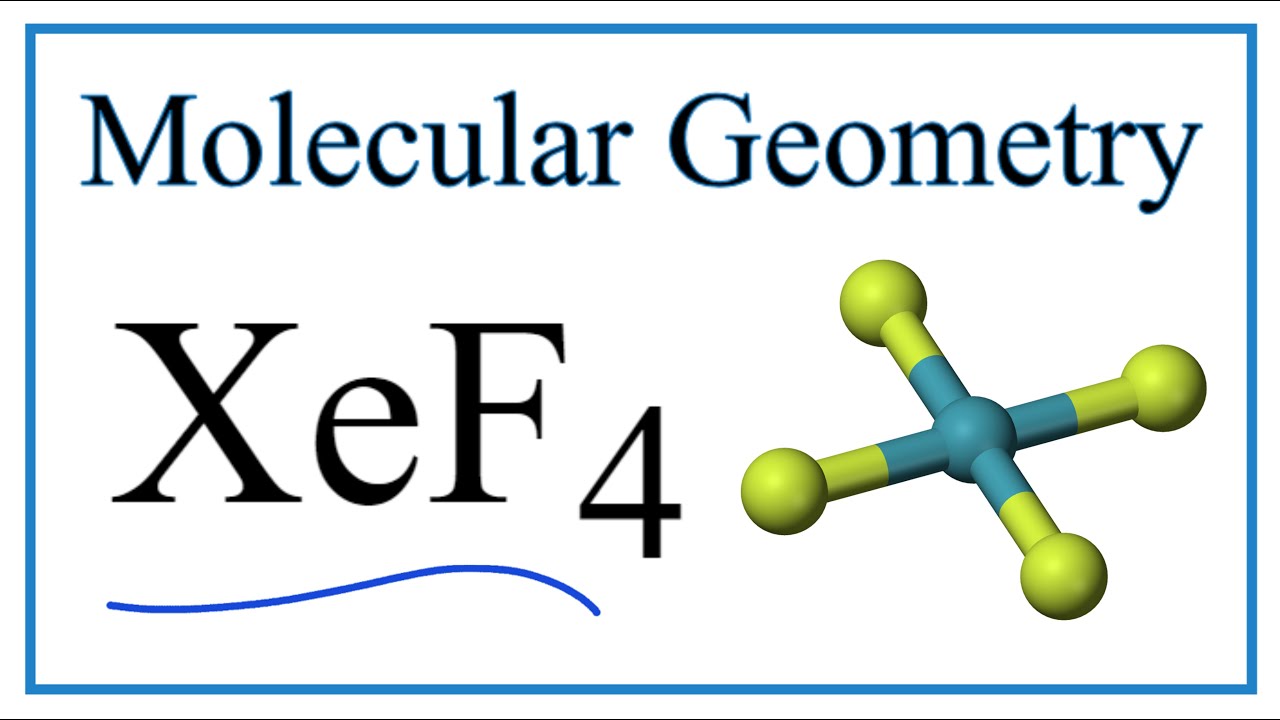
XeF4 Molecular Geometry, Bond Angles & Electron Geometry YouTube
Its structure was determined by both NMR spectroscopy and X-ray crystallography in 1963. [6] [7] The structure is square planar, as has been confirmed by neutron diffraction studies. [8] According to VSEPR theory, in addition to four fluoride ligands, the xenon center has two lone pairs of electrons. These lone pairs are mutually trans. Synthesis

So far, we’ve used eight of the XeF4 Lewis structure’s total 8
328 39K views 3 years ago An explanation of the molecular geometry for the XeF4 (Xenon tetrafluroide) including a description of the XeF4 bond angles. The electron geometry for the Xenon.
Solved
Drawing the Lewis structure of XeF4 involves following a few steps. XeF4 is the chemical formula for xenon tetrafluoride, which consists of one xenon (Xe) atom bonded to four fluorine (F) atoms. Step-by-Step Guide to Drawing the Lewis Structure of XeF4 1. Count the total number of valence electrons

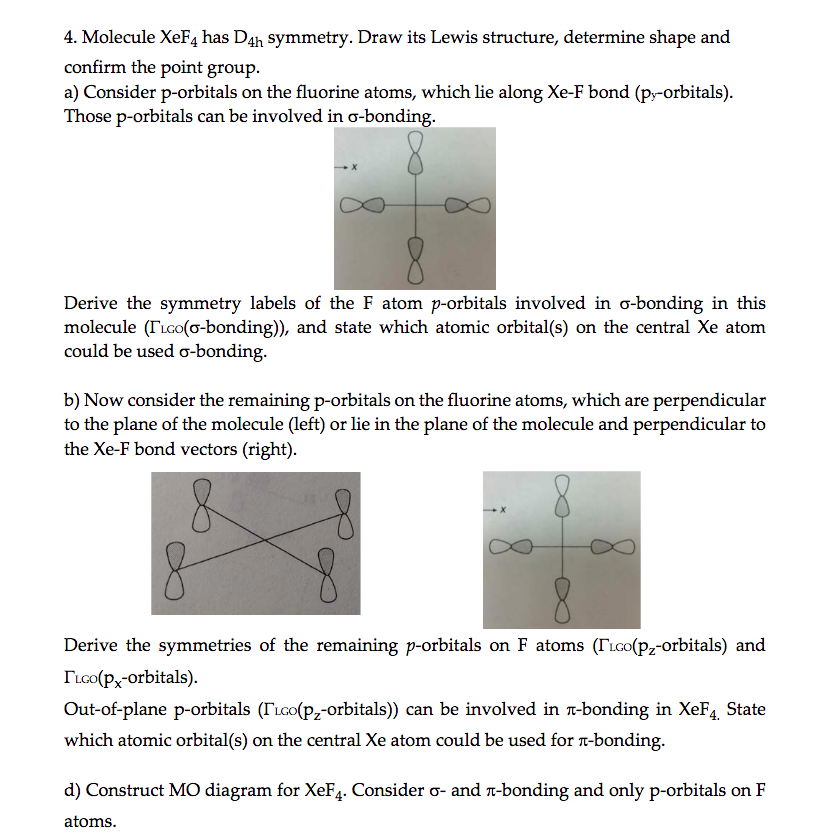
Solved Molecule XeF4 has D4h symmetry. Draw its Lewis
In XeF 4 (Xenon tetrafluoride) lewis structure, there are four sigma bonds and two lone pairs around xenon atom. Each fluorine atom has three lone pairs. In this tutorial, we will learn how to draw lewis structure of XeF 4 step by step. Lewis structure of XeF 4

XeF4 Lewis Structure How to Draw the Lewis Structure for XeF4 YouTube
In the XeF 4 Lewis structure, there are four single bonds around the xenon atom, with four fluorine atoms attached to it. Each fluorine atom has three lone pairs, and the xenon atom has two lone pairs. XeF4 Lewis Structure - How to Draw the Lewis Structure for XeF4 Watch on Contents Steps #1 Draw a rough skeleton structure
Molecular Geometry of XeF4 [with video and free study guide]
Hello Guys!Today we are going to look at the Lewis Structure of XeF4 ( Xenon Tetrafluoride )Although Xenon is a noble gas it reacts with four Fluorine atoms.
Molecular Geometry Of Xef4 Youtube
The Lewis structure for XeF 4 requires you to place more than 8 valence electrons on Xe.. Let's do the XeF4 Lewis structure. Xenon has 8 valence electrons. Fluorine has 7, but we have four of the Fluorines; so that gives us 8 plus 28: 36 valence electrons. We'll put Xenon in the center, it's the least electronegative; and then Fluorines on.
Solved 4. Molecule XeF4 has Dsh symmetry. Draw its Lewis
Xenon tetrafluoride (XeF4) is a square planar, non-polar molecule. The Xenon atom has 4 bonding pairs of electrons and 2 lone (non-bonding) pairs of electro.
[Solved] Complete the chart. Molecular Polarity. XeF4 +Lewis Structure
Chemistry tutorial for the Lewis dot structure and molecular geometry of xenon tetrafluoride (XeF4).
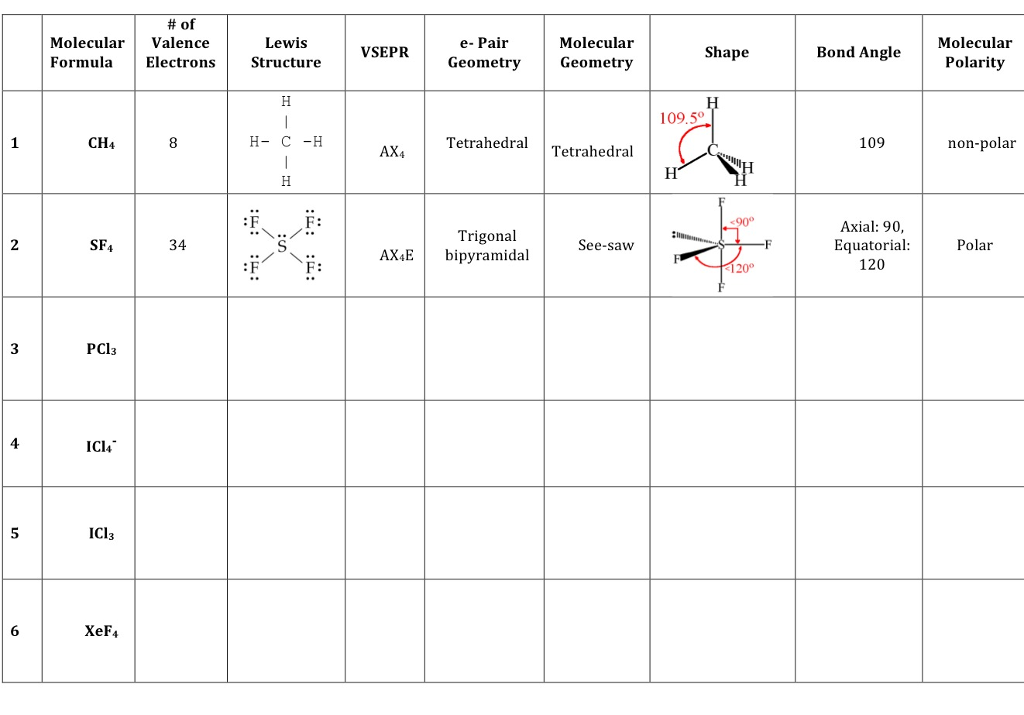
Solved Of Molecular Valence Formula Electrons CH4 SF4 34
XeF4 Lewis Structure, Molecular and Electron Geometry based on the VSEPR theory, the steric number, Hybridization and expected bond angles.
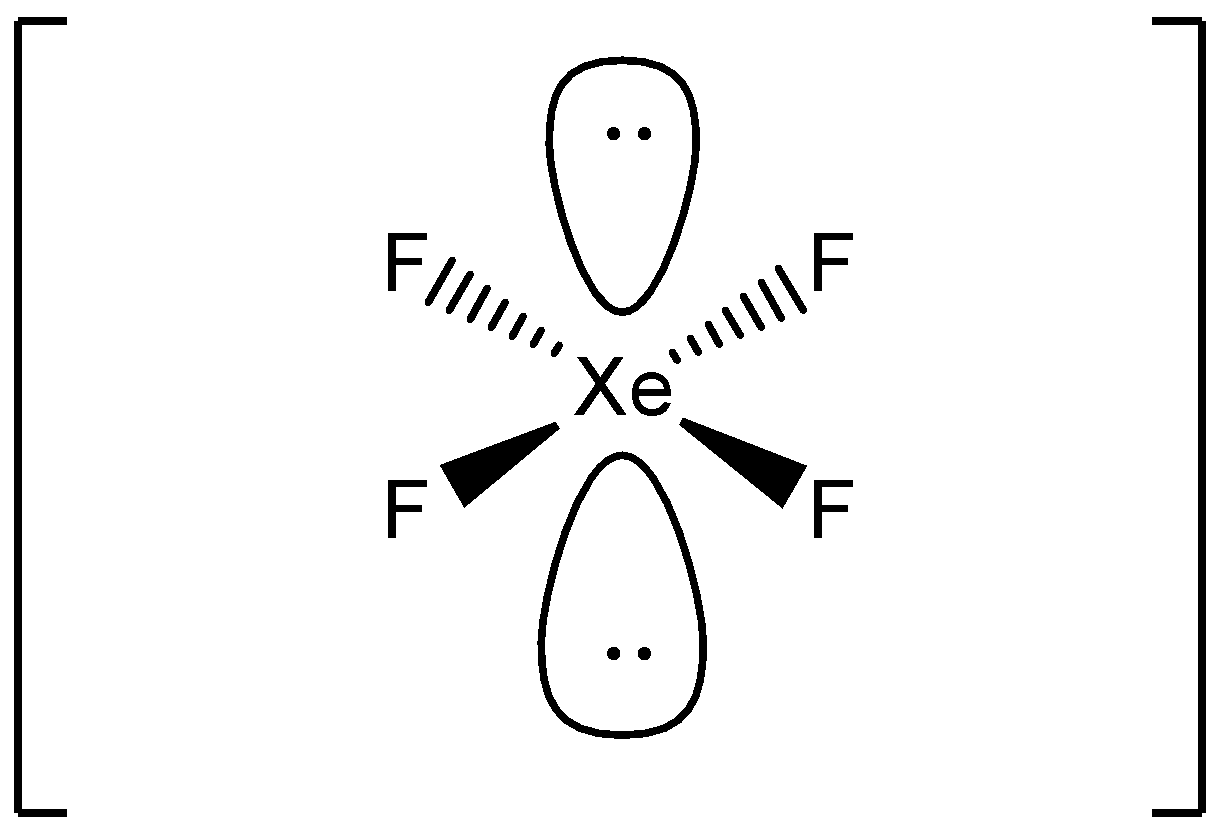
Xef4 Vsepr
The first step is to sketch the Lewis structure of the XeF4 molecule, to add valence electrons around the xenon atom; the second step is to add valence electrons to the four fluorine atoms, and the final step is to combine the step1 and step2 to get the XeF4 Lewis Structure.