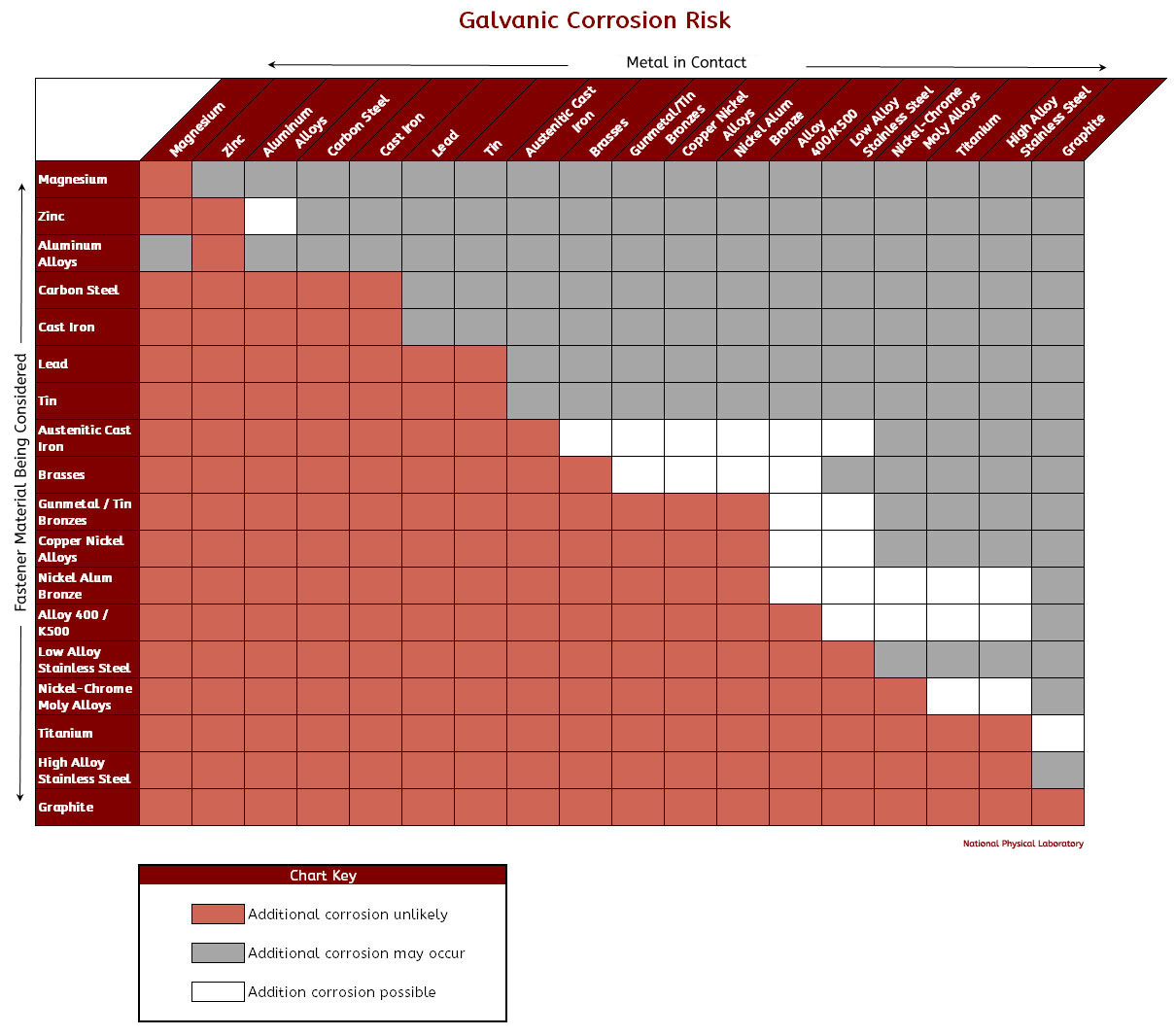
Galvanic Corrosion Chart
up a galvanic action which results in the deterioration of one of them. The following is a list of the more common commercial metals, sequenced according to what is known as the "Galvanic Table": THE GALVANIC TABLE 1. Aluminum 2. Zinc 3. Steel 4. Iron Anodic or Active (+) 5. Nickel 6. Stainless Steel Series 400 ↔ 7. Tin 8. Lead 9. Brass 10.

Mixing Metals in Fasteners
The galvanic series determines the electrochemical potential and nobility of metals and metal alloys. Corrosivity Each alloy or metal has a distinctive corrosion potential. The more negative a metal or alloy is, the more likely it is to suffer galvanic corrosion.

Galvanic Corrosion Chart Pay attention! You might accidentally learn
Simply speaking, galvanic corrosion is the damage or deterioration of metal that takes place between dissimilar metals because of an electrochemical reaction.
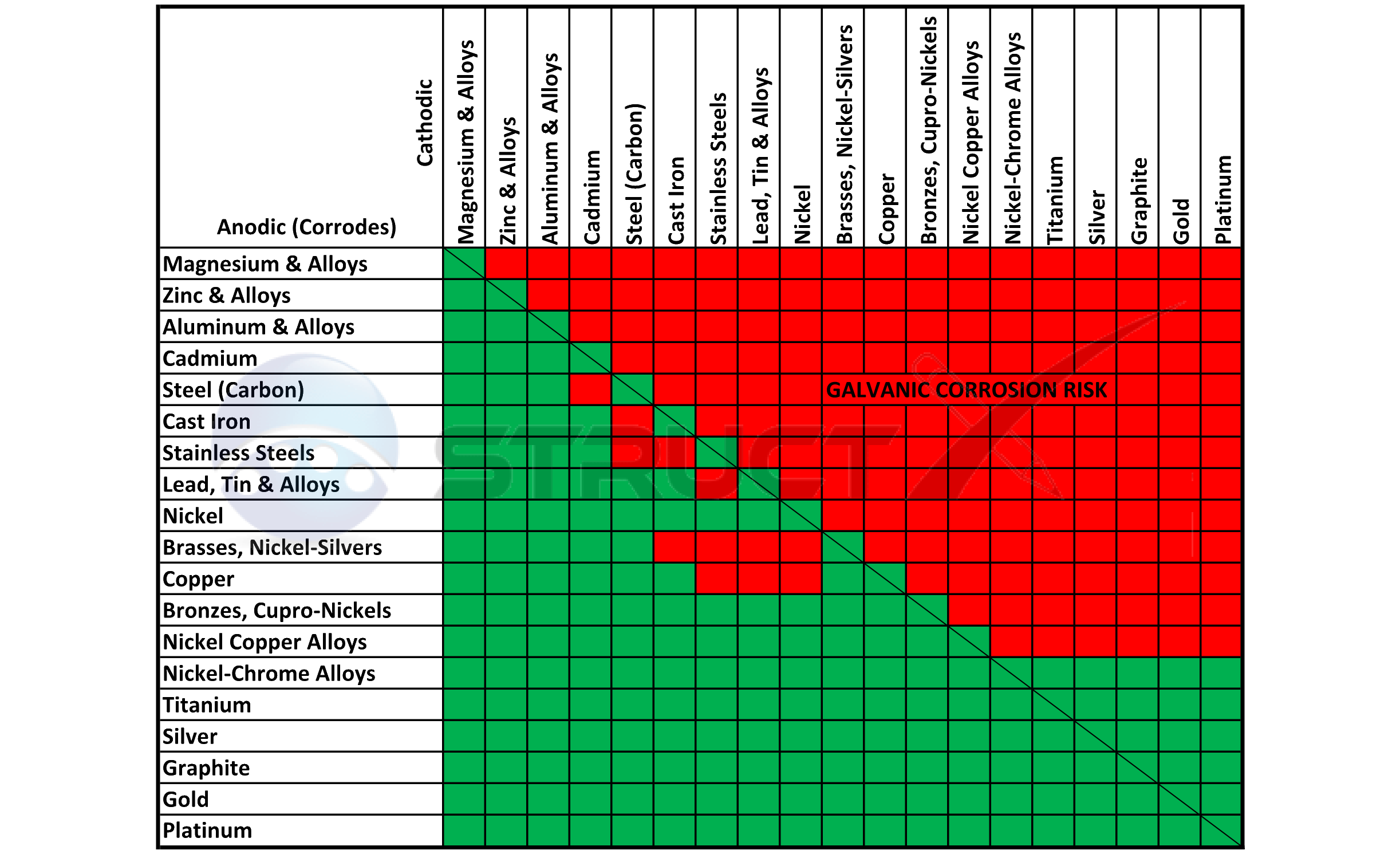
Galvanic Series (electrochemical series)
Galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte.

GALVANICCORROSIONTABLE CMP Products Limited
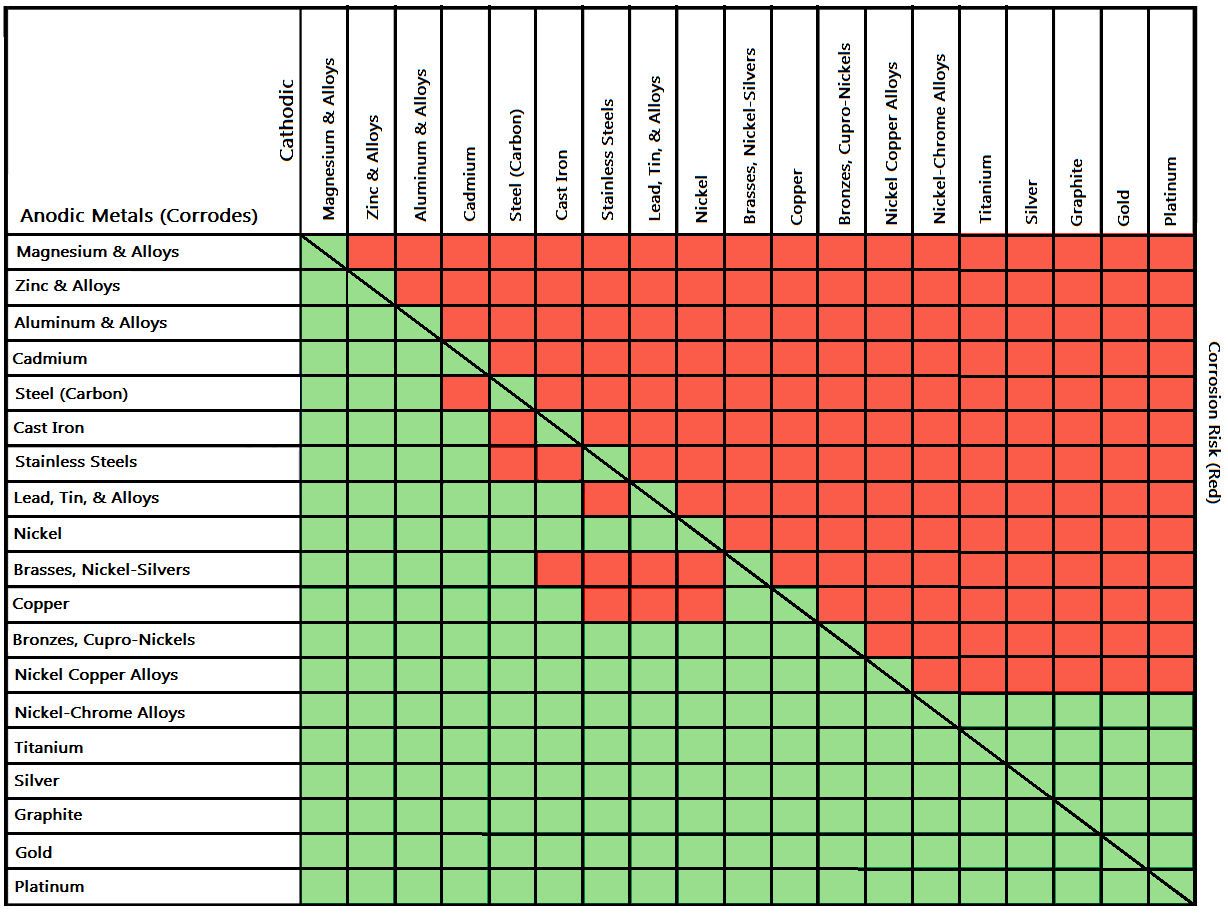
Galvanic/Dissimilar Metal or Bimetallic Corrosion is a type of electrochemical corrosion, where a material corrodes if it comes in contact with another material in the presence of an electrolyte. During product design, engineers should select material ensuring the dissimilar metal corrosion has the minimum or a positive impact on product function.
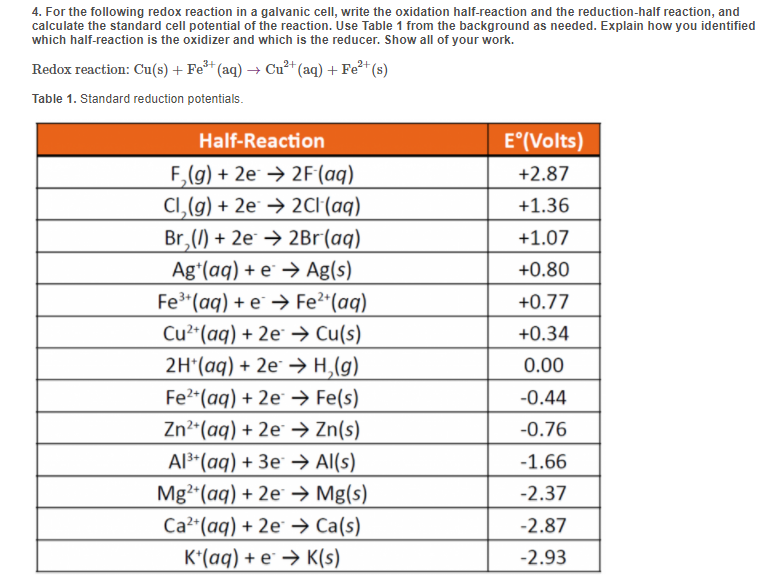
Solved 4. For the following redox reaction in a galvanic
Galvanic Table The following galvanic table lists metals in the order of their relative activity in seawater environment. The list begins with the more active (anodic) metal and proceeds down the to the least active (cathodic) metal of the galvanic series.
Galvanic Chemistry Dictionary & Glossary
The Galvanic Series, also called the electro-potential series, lists metals in the order of their nobility. (Noble metals are those that are resistant to corrosion and oxidation.) When two metals are immersed in an electrolyte, while also being connected externally by a conductor, the less noble metal experiences galvanic corrosion.

Galvanic Corrosion PDF Corrosion Stainless Steel
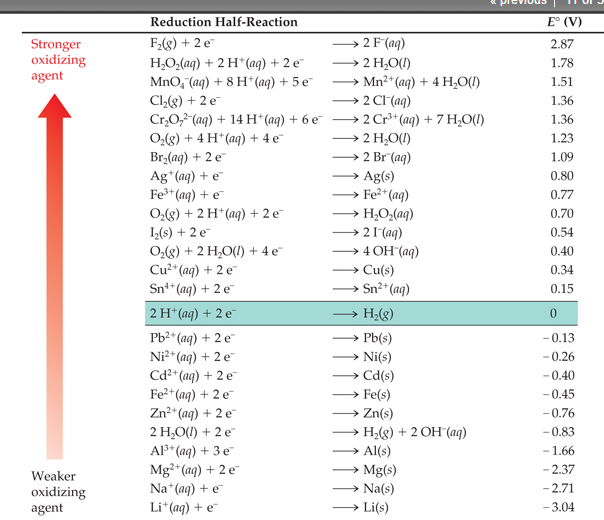
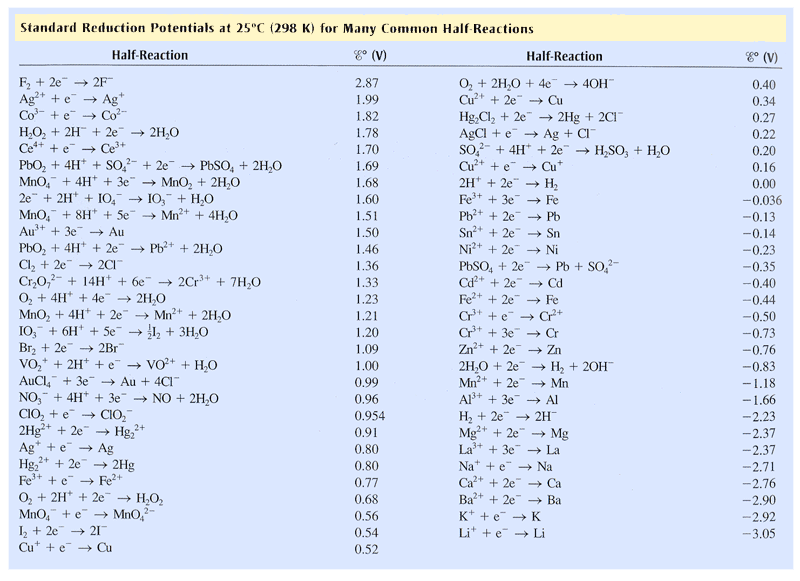
Corrosion potential and the galvanic series. When a metal corrodes in an electrolyte, atoms from the metal separate into ions and electrons (e - ), with the ions dissolving into the electrolyte. For example, for iron (Fe) the reaction is: Fe → Fe 2+ + 2e −. This is called an anodic reaction and Fe 2+ ions are formed.

Galvanic Reaction Chart All Points Fasteners
Below is a galvanic reaction chart for dissimilar metals. Please understand that green represents "lower risk" not "no risk." It should be noted that if sacrificial plating is incorporated in the fastener design, then galvanic action can result in the deterioration of the sacrificial coating, rather than of the fastener.
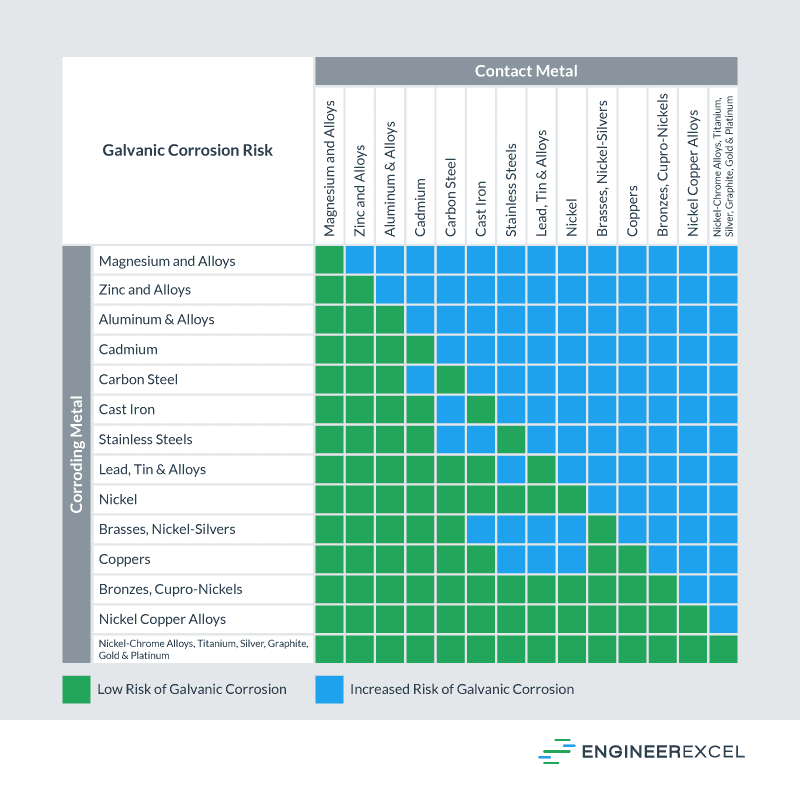
Galvanic Corrosion [with Chart] EngineerExcel
The table is the galvanic series of metals in sea water from Army Missile Command Report RS-TR-67-11, "Practical Galvanic Series." The Galvanic Table Active (Anodic) Magnesium Mg alloy AZ-31B Mg alloy HK-31A Zinc (hot-dip, die cast, or plated) Beryllium (hot pressed) Al 7072 clad on 7075 Al 2014-T3 Al 1160-H14
Galvanic Potential Chart
Shop Like A Billionaire, Come & Check Everything At A Surprisingly Low Price. Come and check everything at a surprisingly low price, you'd never want to miss it.
Galvanic Corrosion Common Questions Answered
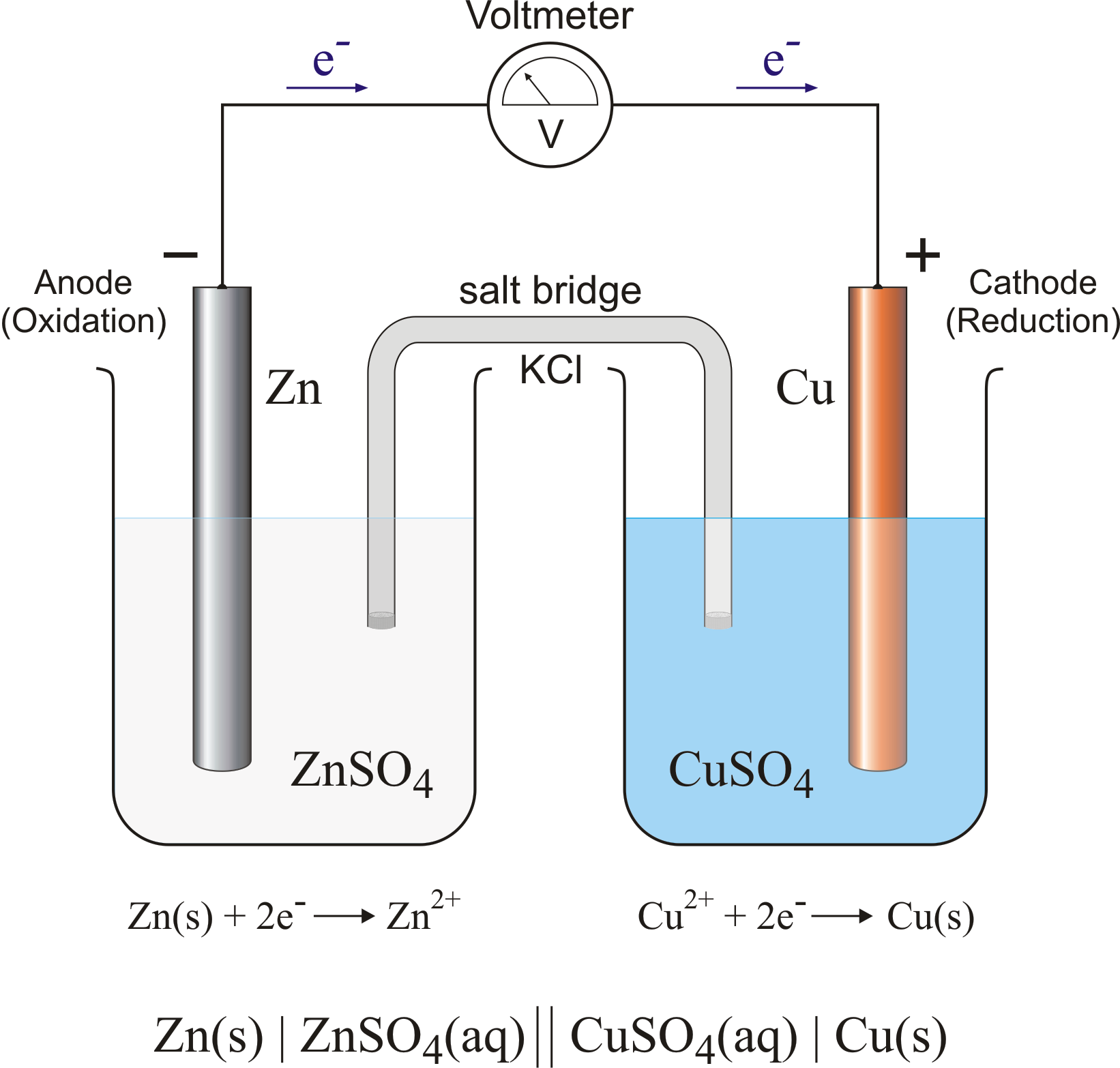
Draw (graphically) a galvanic cell that takes advantage of the spontaneous redox reaction indicated in Q2. Make you indicate all of the following components and aspects in your cell drawing: Anode. Cathode. Electrode Salt Bridge. Wire. Voltmeter. Oxidation half reaction. Reduction half reaction.
Solved Consider The Following Galvanic Cells. For Each Ga...
Galvanic corrosion describes a process in which two (or more) dissimilar metals are used together, resulting in a corrosive process. A common application that may experience galvanic corrosion is using an attachment, such as a bolt, that is of a different metal than the primary structure, such as a beam. Table of Contents

Separating Galvanic Metals JLC Online
Print. ResearchGate. (2014, March). Galvanic series of various materials in flowing seawater (2.5-4 m/s) at temperatures in the range from 5 to 30oC. [ upload Retrieved from https://www.researchgate.net/figure/Galvanic-series-of-various-materials-in-flowing-seawater-25-4-m-s-at-temperatures-in_fig1_260528991
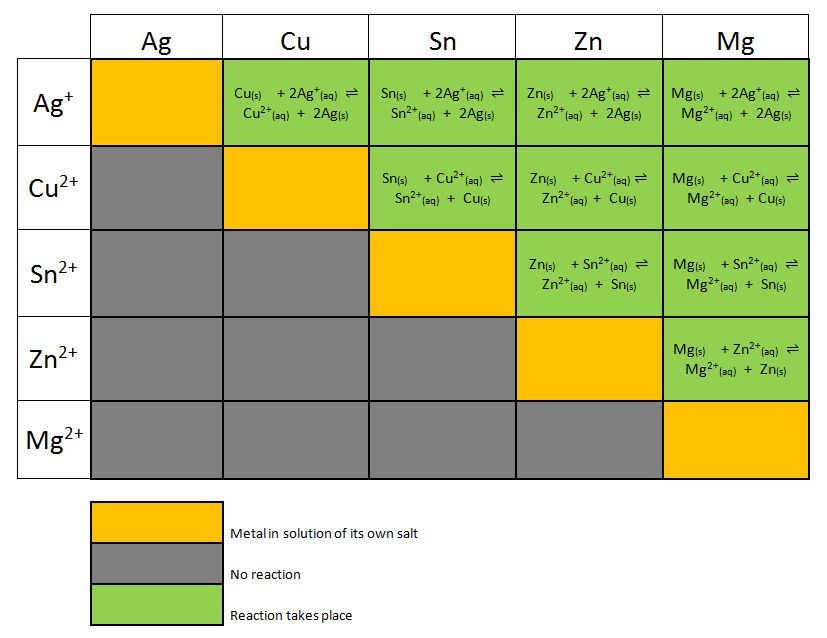
Electrochemistry Galvanic Cells and the Nernst Equation
Essentially, galvanic corrosion occurs when two different metals immersed in an electrolyte are joined together. In this scenario, the base or the metal with lesser nobility will undergo corrosion. Thus, the corrosion rate can be determined based on the nobility of metals and the electrolyte to which they're exposed. Advertisement

The Galvanic Series the essential guide EngineeringClicks
75 of The Top 100 Retailers Can Be Found on eBay. Find Great Deals from the Top Retailers. eBay Is Here For You with Money Back Guarantee and Easy Return. Get Your Galvanic Today!