
How many valence electrons are in an atom of Phosphorus MakeTheBrainHappy
Drawing Bohr-Rutherford diagrams is super easy using the following steps: Find the number of protons, neutrons and electrons for the atom. The number of protons is the atomic number. The number of neutrons can be found by subtracting the number of protons from the atomic mass rounded to the nearest whole. This is because protons and neutrons.
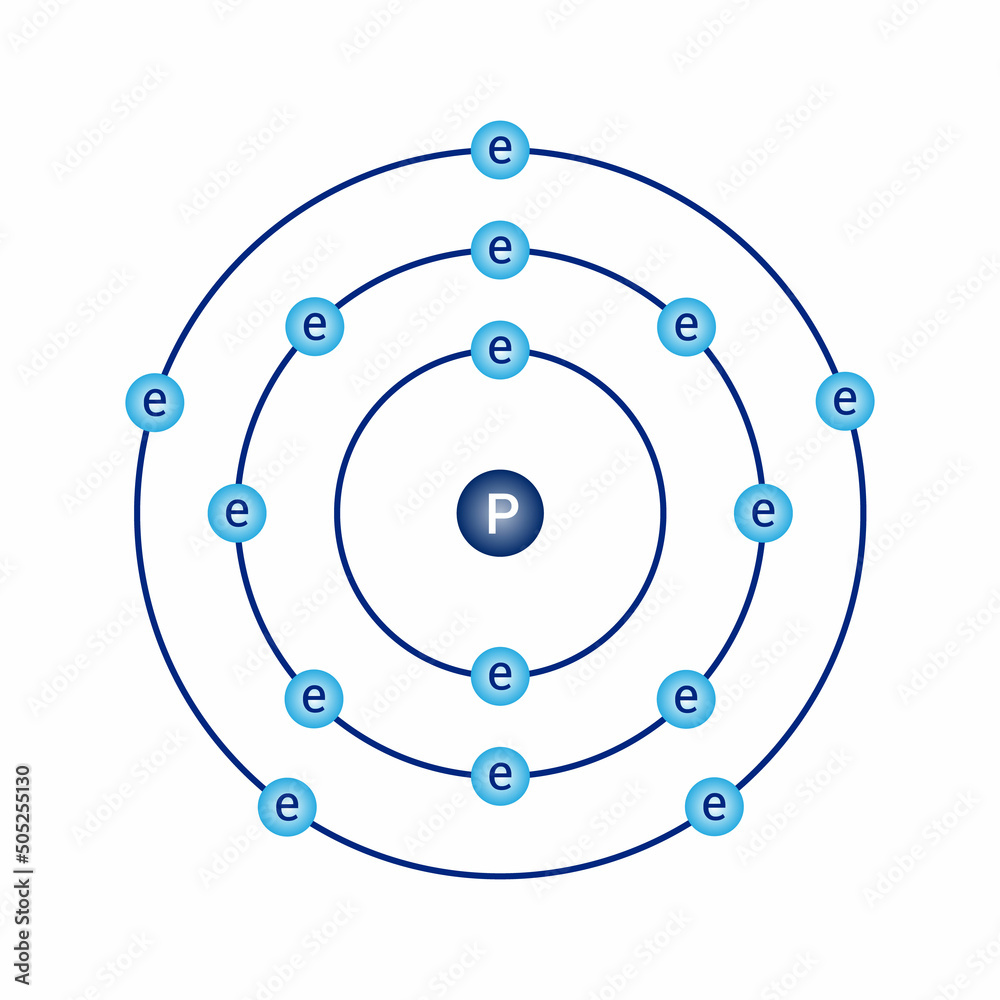
Phosphorus Bohr Diagram
Bohr model of all Elements is mentioned in the chart below.. Phosphorus (P) 2, 8, 5: 16: Sulfur (S) 2, 8, 6: 17: Chlorine (Cl) 2, 8, 7: 18: Argon (Ar) 2, 8, 8: 19: Potassium (K) 2, 8, 8, 1: 20:. Orbital Diagram of All Elements (Diagrams given Inside) Subscribe to our newsletter. Subscription Form.

Bohr Model Phosphorus Atom Electron Structure Stock Vector (Royalty
We explain how the Bohr atomic model works and provide example Bohr diagrams. Call Direct: 1 (866) 811-5546 Sign In Start Free Trial. the Rutherford Model. The Bohr Model has an atom with a positively-charged nucleus surrounded by negatively-charged electrons that have circular, planetary-like orbits.. Phosphorus (P) 15: 15: 16: Sulfur (S.
Diagrama De Bohr Tabla
How to draw the Bohr-Rutherford Diagram for Phosphorous. 2 electrons can go in the first shell, 8 in the second, 8 in the third, and so on.
Phosphorus Bohr Diagram
In this video we'll look at the atomic structure and Bohr model for the Phosphorus atom (S). We'll use a Bohr diagram to visually represent where the electro.

Pin on phosphorus
The Bohr model of the hydrogen atom (Z = 1) or a hydrogen-like ion (Z > 1), where the negatively charged electron confined to an atomic shell encircles a small, positively charged atomic nucleus and where an electron jumps between orbits, is accompanied by an emitted or absorbed amount of electromagnetic energy (hν). The orbits in which the electron may travel are shown as grey circles; their.
Bohr model diagram of phosphorus P in atomic physics vector de Stock
Find step-by-step Biology solutions and your answer to the following textbook question: (a) Draw the Bohr-Rutherford diagram (without neutrons) for an atom of each of the following elements: lithium, oxygen, calcium; and phosphorus. (b) Draw the; Bohr-Rutherford diagram (without neutrons) for the ion formed by each of the elements in (a). (c) Write the chemical symbol for each ion.

Phosphorus Definition, Facts, Symbol, Discovery, Property, Uses
Identification of elements using Bohr-Rutherford Diagrams. Learn with flashcards, games, and more — for free.

Draw the structure of phosphorus atom according to Bohr’s model of atom
Figure \(\PageIndex{1}\): A Bhor's model can be used to diagram the location of electrons in each energy shell for an atom. Notice that protons go in the nucleus of the atom and electrons are drawn on orbits surrounding the nucleus. Image from Wikimedia commons. Example \(\PageIndex{2}\) Draw the Bohr's model for sodium (Na).
PPT SNC2D CHEM UNIT REVIEW PowerPoint Presentation, free download
(a) Draw the Bohr-Rutherford diagram (without neutrons) for an atom of each of the following elements: lithium, oxygen, calcium; and phosphorus. (b) Draw the; Bohr-Rutherford diagram (without neutrons) for the ion formed by each of the elements in (a). (c) Write the chemical symbol for each ion.

Phosphorus Bohr Model — Diagram, Steps To Draw Techiescientist
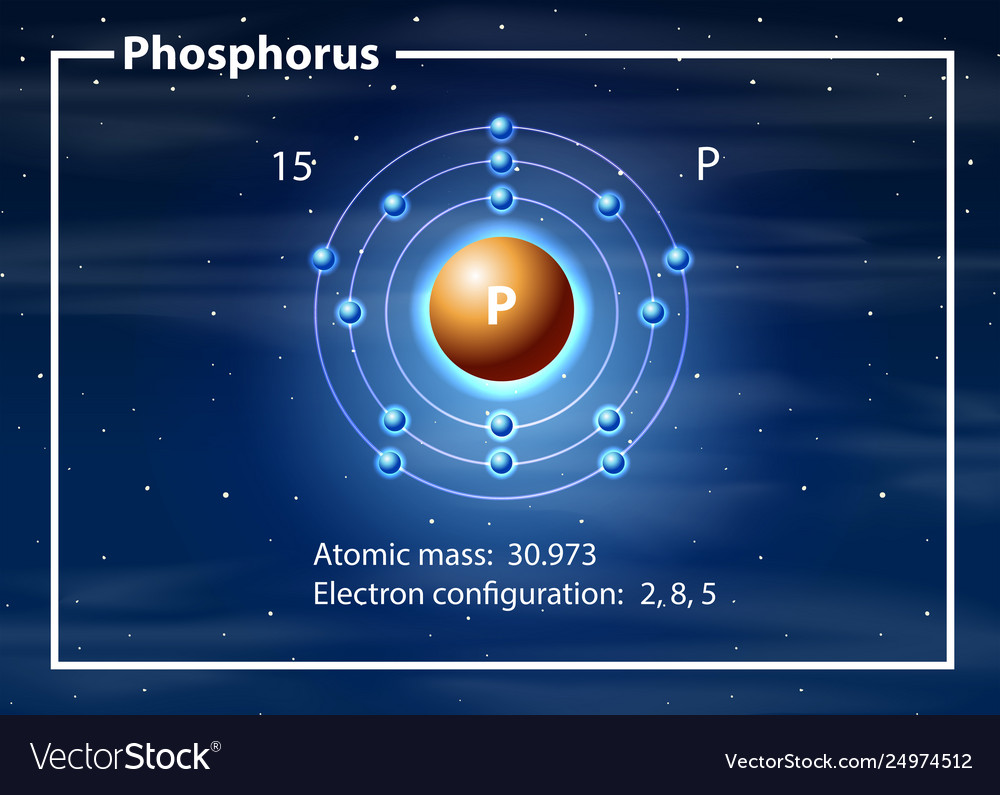
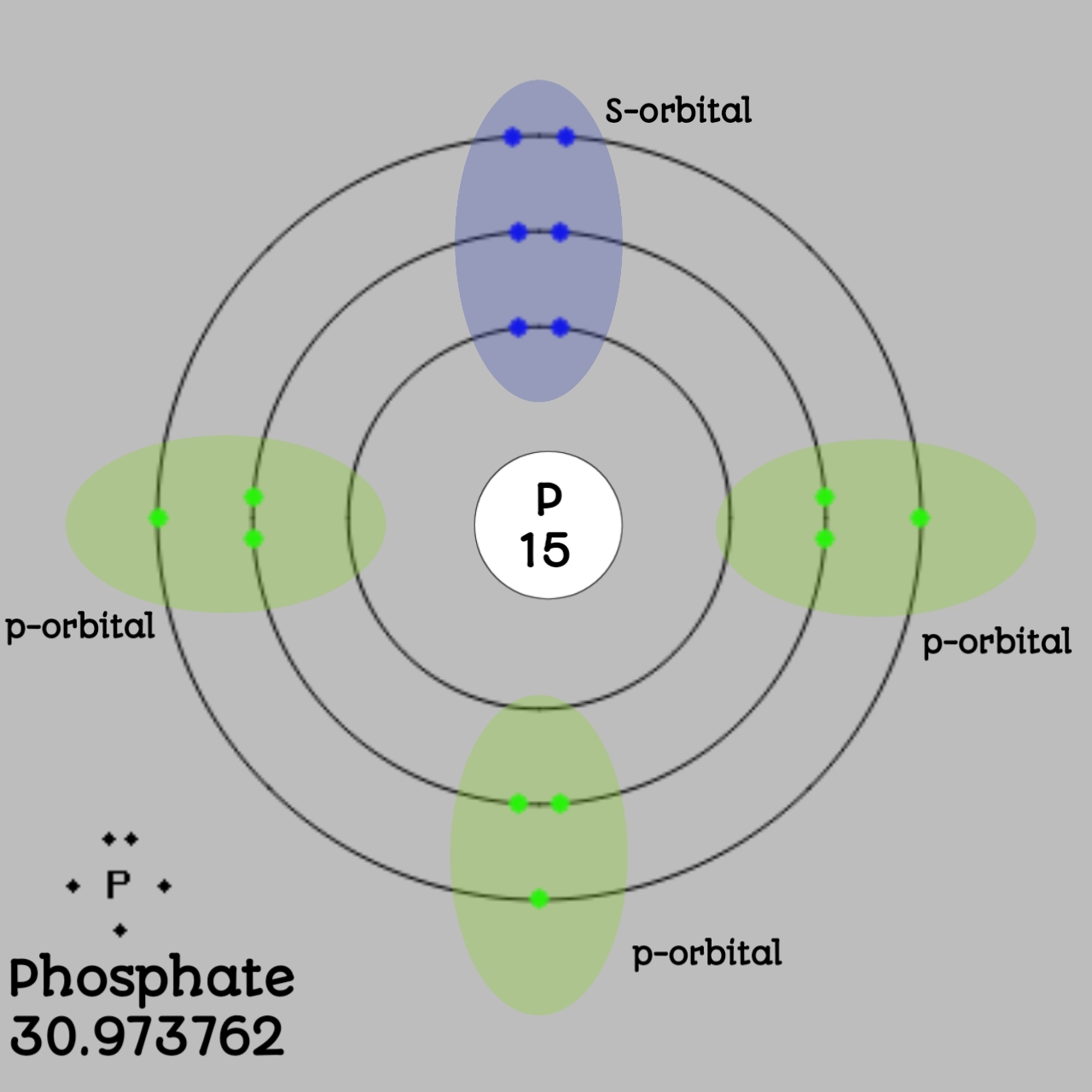
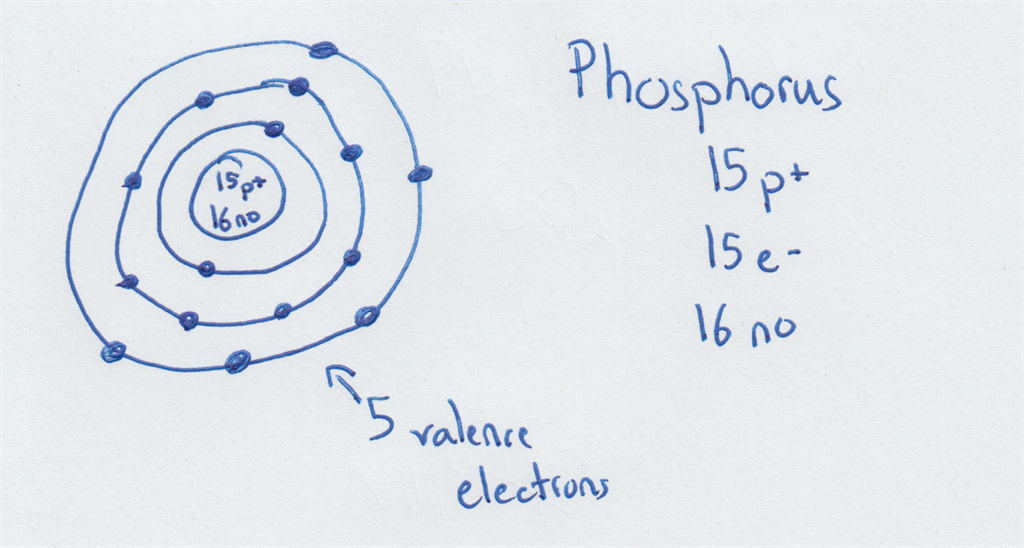
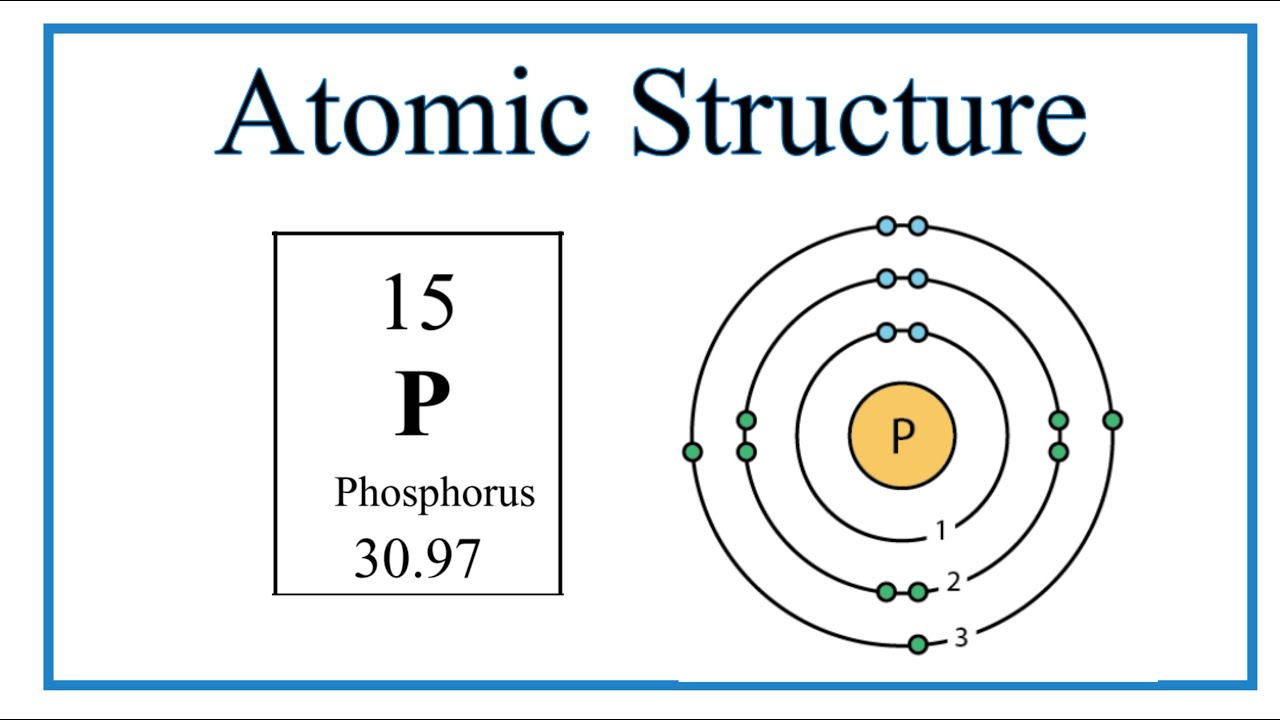
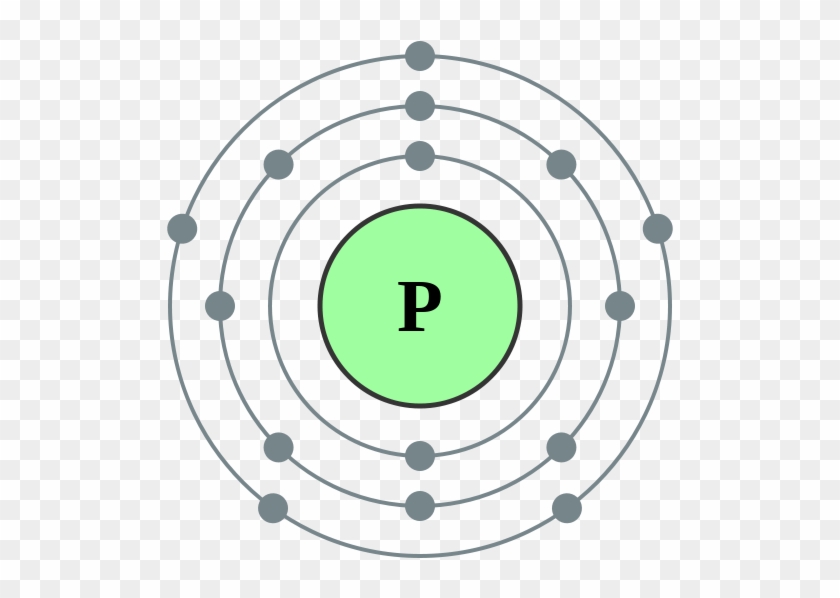
The Bohr Model of Phosphorus(P) has a nucleus that contains 16 neutrons and 15 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Phosphorus contains 5 electrons that also called valence electrons.

Bohr Model Example Phosphorus Diagram Quizlet
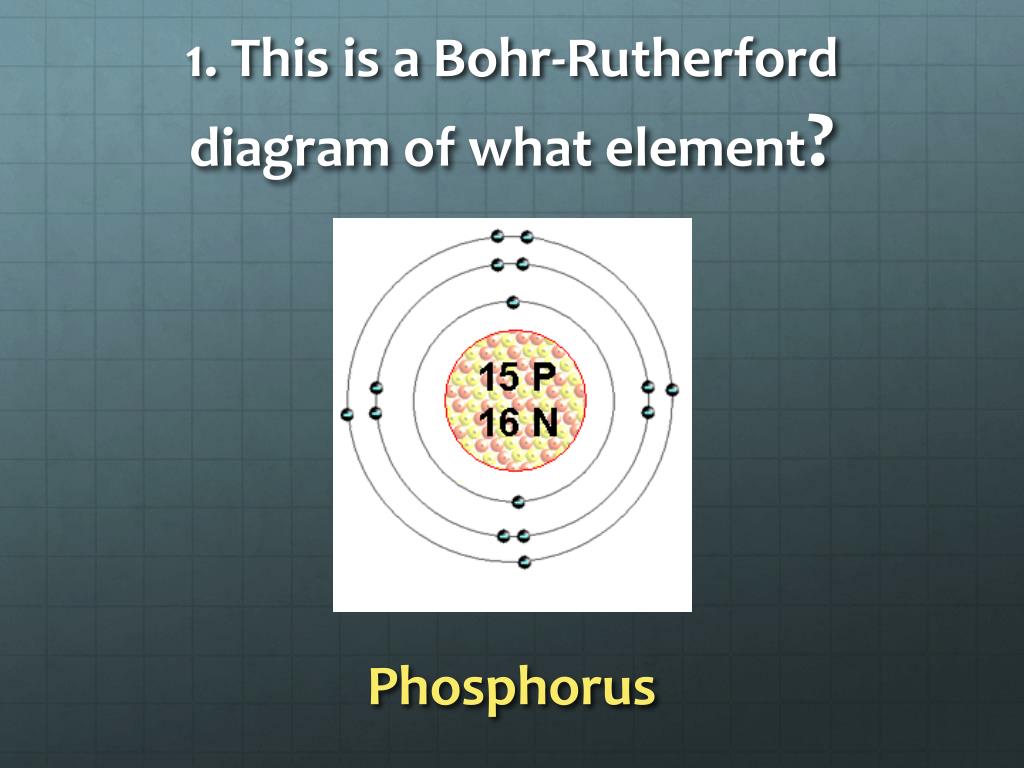
4) The following diagram is a Bohr-Rutherford diagram of one element from the periodic table: To which group and period does this element belong? A) Period 3 group 4. B) Period 4 group 4. C) Period 3 group 1. D) Period 1 group 3. 5) Referring to the periodic table, find an element that has the same number of electron shells and
Phosphorus Bohr Diagram
Numerous models of the atom had been postulated based on experimental results including the discovery of the electron by J. J. Thomson and the discovery of the nucleus by Ernest Rutherford. Bohr supported the planetary model, in which electrons revolved around a positively charged nucleus like the rings around Saturn—or alternatively, the.
draw your own bohr model for dickson only Tutor help Now
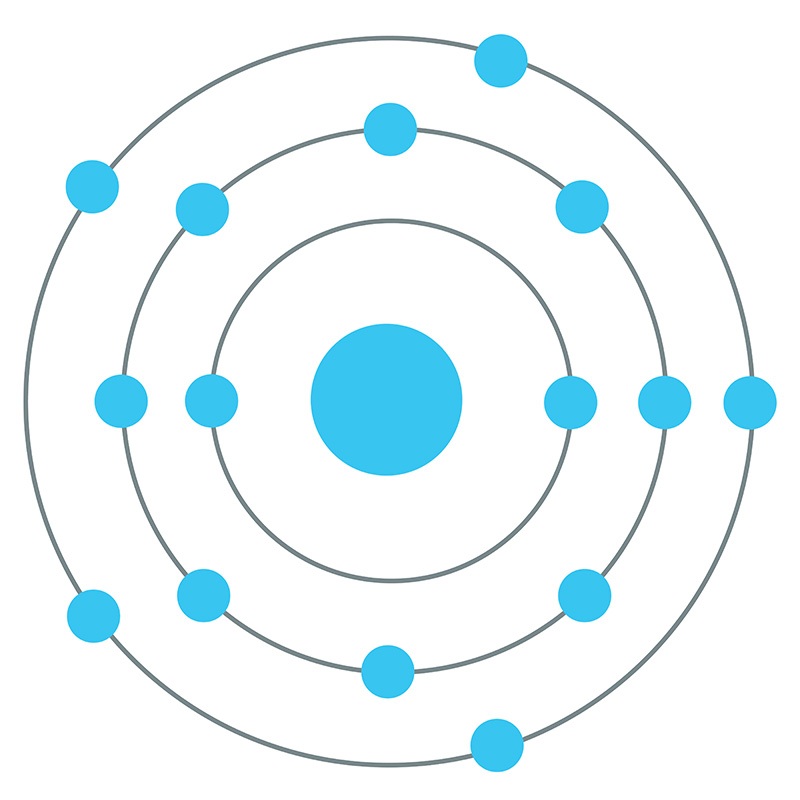
The Bohr - Rutherford Diagram also shows the total number of electrons is 15. Recall: The number of protons = number of electrons. The electrons occur in three orbits. Two electrons are in Orbit 1, eight electrons are in Orbit 2 and five electrons are in Orbit 3. The outer orbit (i.e., valence) is not full. Thus, phosphorus will react
Atomic Structure (Bohr Model) for Phosphorus (P) YouTube
The bohr Rutherford diagram for oxygen has 8 protons and 8 neutrons. There are 2 electrons on the first orbital and six on the second. The bohr Rutherford diagram for oxygen has 8 protons and 8.
Bohr Model Of A Phosphorus Atom Electron Configuration Of Sodium
The electrons occur in three orbits. Two electrons are in Orbit 1, eight electrons are in Orbit 2 and five electrons are in Orbit 3. The outer orbit (i.e., valence) is not full. Thus, phosphorus will react with other elements to reach the maximum. Example 2: Fluorine This is the Bohr -Rutherford Diagram for Fluorine (Atomic Number 9).