
Question Video Describing How Enzymes Affect Biochemical Reactions
Some experts believe that honey when honey comes in contact with metals like copper or iron (commonly found in spoons) these metals are capable of destorying beneficial enzymes. Metals like copper and iron react with the acids in honey to produce salts. This process kills off useful enzymes. Copper is known to react with acids in honey.
bolita Último Pisoteando anatomia apis mellifera suéter puente Condensar
2.1. Antioxidant Activity of Tested Honeys. Honey serves as a source of natural antioxidants, which play an important role in food preservation and human health by combating damage caused by oxidizing agents, namely reducing the risk of heart disease, cancer, immune-system decline, cataracts, different inflammatory processes, etc. [32,33,34].There is no official method for honey antioxidant.
Enzyme Cofactors
The pH scale of honey is normally between 3.4 to 6.1 and acidic substances can corrode metals and it is often feared that metal components can be mixed in honey, such as metal spoons or other metal utensils. So is it bad to use metal spoon with honey? In respect to this, not all honey has the same level of acidity.

Honey Lemon Enzyme Recipe
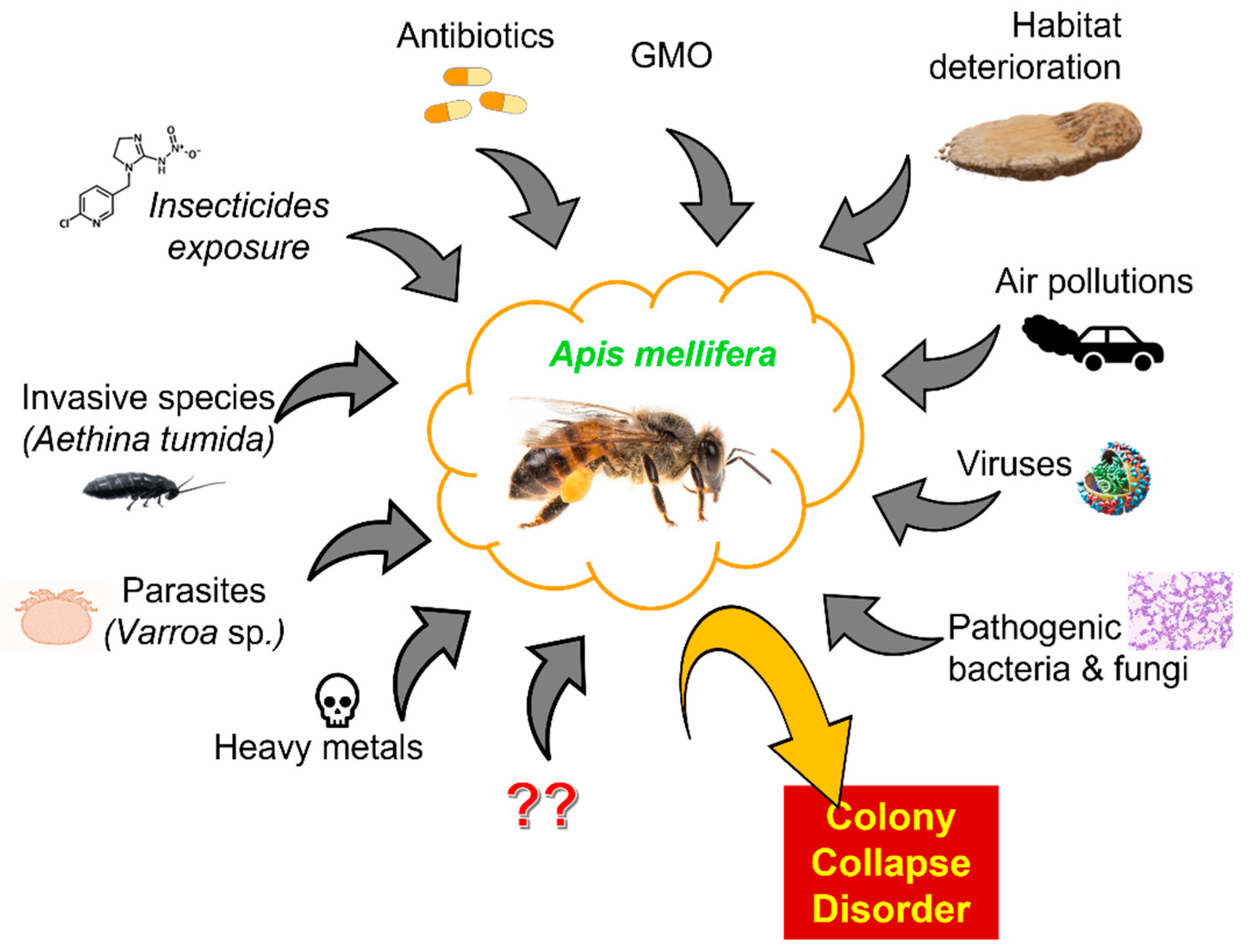
All Bees Do Not Produce Honey. One of the most common misconceptions about raw honey is that it can come from any type of bee. It is estimated that out of 20,000 known species of bees in the world, only 5% are able to produce edible honey. The only kind of bee that can make it is the honeybee. The honeybee species is one that lives in large.
Rhomboid Protease Enzyme Photograph by Laguna Design Pixels
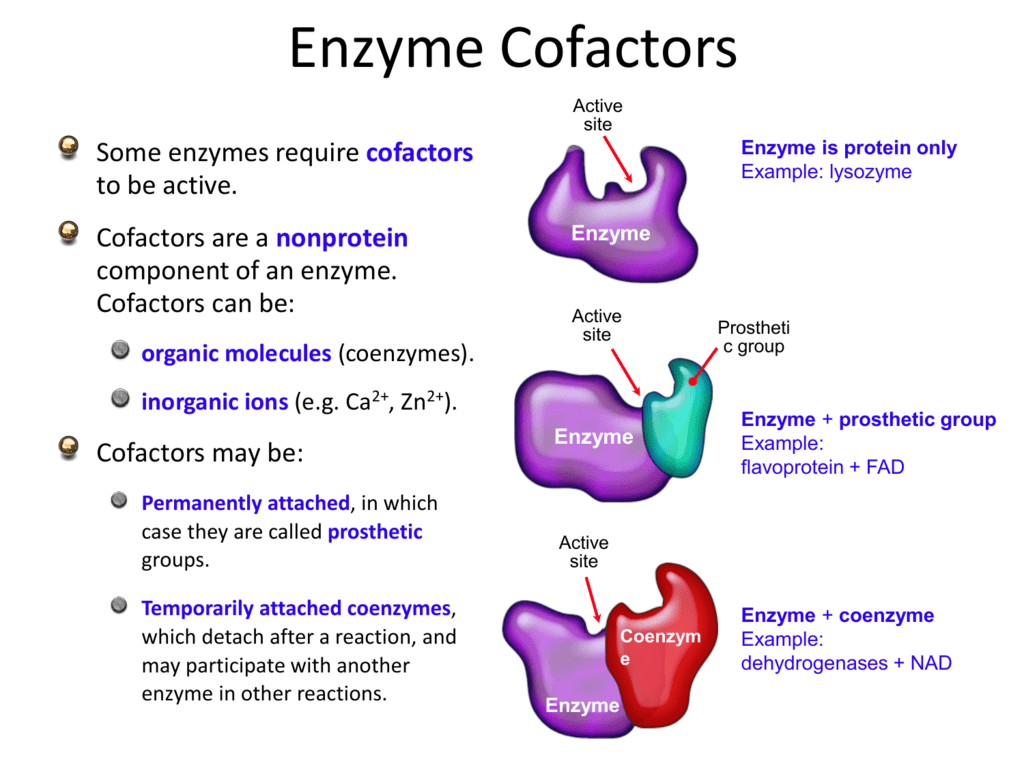
Honey is known for its content of biomolecules, such as enzymes. The enzymes of honey originate from bees, plant nectars, secretions or excretions of plant-sucking insects, or from microorganisms such as yeasts. Honey can be characterized by enzyme-catalyzed and non-enzymatic reactions. Notable examples of enzyme-catalyzed reactions are the production of hydrogen peroxide through glucose.

Pin on Healthy
This is why you should avoid metal. Because honey is acidic and reacts poorly with metal, that's why the substance can't be kept in a metal container. While corrosion is a factor, it isn't the only issue with the pairing; ultimately, metal causes honey to oxidize. According to Kemin, that means the honey will develop "off-flavors and off-odors."

Did you know that... there are living enzymes in honey? Did you know
Honey is known for its content of biomolecules, such as enzymes. The enzymes of honey originate from bees, plant nectars, secretions or excretions of plant-sucking insects, or from microorganisms such as yeasts. Honey can be characterized by enzyme-catalyzed and non-enzymatic reactions. Notable examples of enzyme-catalyzed reactions are the.

Raw Honey Enzymes Raw honey, Enzymes, Honey
The theory behind this claim is that the ions present in metal can react with the enzymes in honey, causing them to break down and lose their functionality. However, scientific research on this topic has yielded mixed results, with some studies suggesting that metal spoons can indeed affect honey enzymes, while others have found no significant.

Enzymes HARVEST SPOON
The other enzymes in honey are affected similarly. Enzyme activity stops when honey is held at freezing temperatures but returns when warmed back up. It does not return when destroyed by heat. Two interesting side notes are that almost all the enzymes in honey are introduced by the bees, and all break down when liquefying crystallized honey in.

Honey In Spoon Stock Photo Image 4917040
Honey is acidic due to its organic acid content. The pH scale of honey is usually between 3.4 - 6.1. Because acidic substances can corrode metals (such as this iron spoon) it is feared that metal components can be mixed in honey. Like the concept of cooking utensils with acidic ingredients too. Not all honey has the same level of acidity.
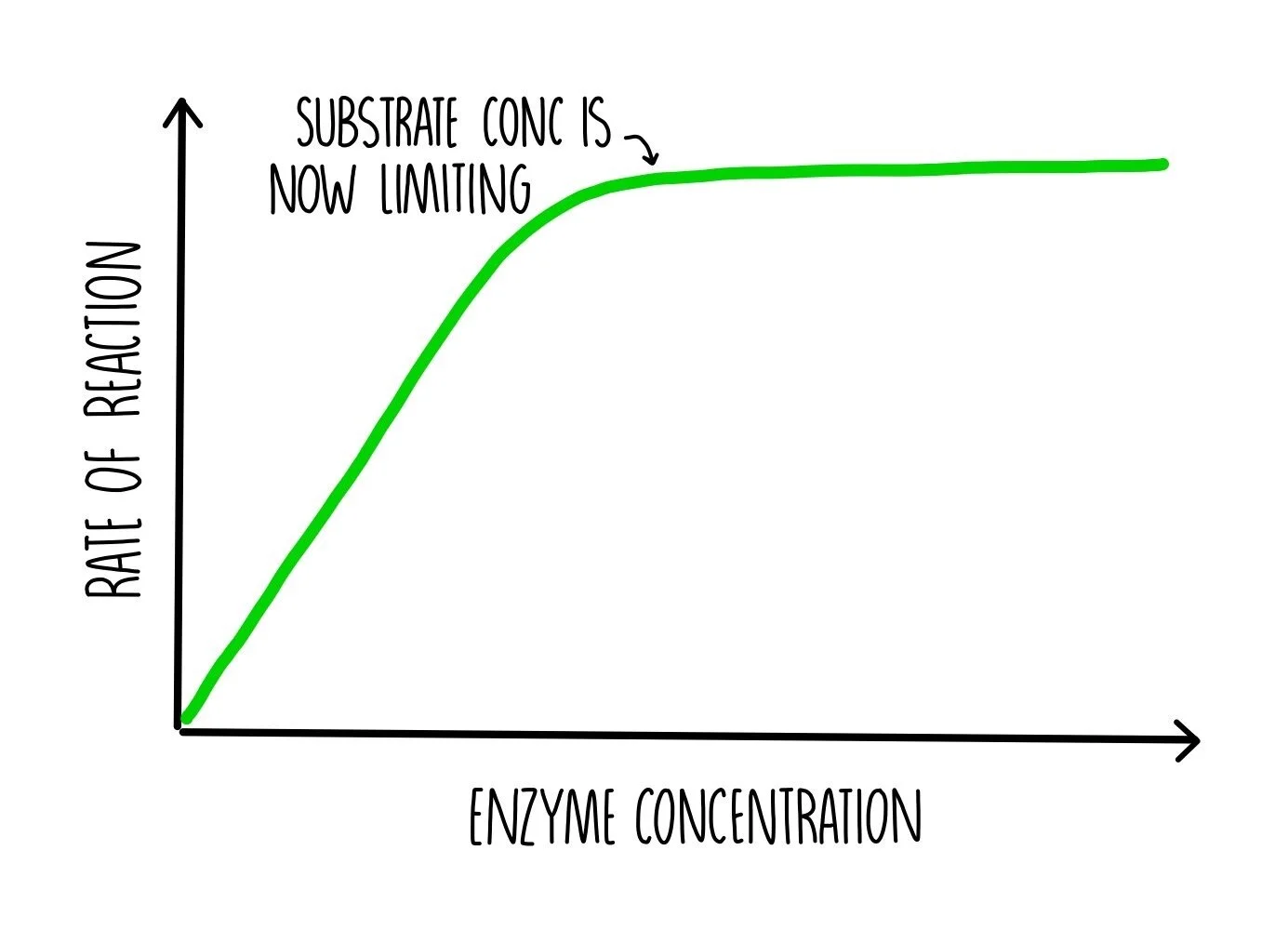
Enzymes (ALevel) — the science sauce
Honey is known for its content of biomolecules, such as enzymes. The enzymes of honey originate from bees, plant nectars, secretions or excretions of plant-sucking insects, or microorganisms such as yeasts. Honey can be characterized by enzyme-catalyzed and non-enzymatic reactions. Notable examples of enzyme-catalyzed reactions are the production of hydrogen peroxide through glucose oxidase.
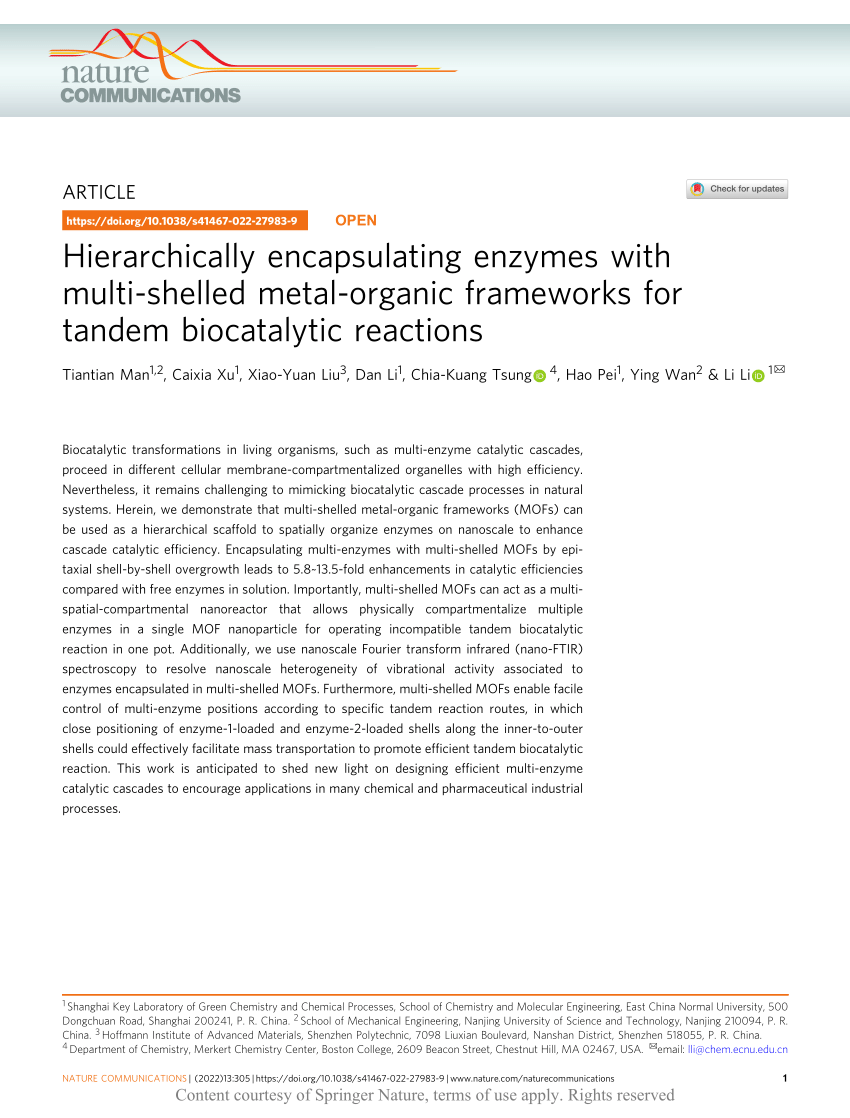
(PDF) Hierarchically encapsulating enzymes with multishelled metal
Honey enzymes originate from three major sources: plant nectars and secretions, honeybees, and excretions of plant-sucking insects. Biochemical reactions can be divided to two types: enzyme-catalyzed and non-enzymatic reactions [ 4 ]. Enzyme-catalyzed reactions in honey are known to affect its quality and biological activities [ 5, 6, 7 ].
Bread with Honey Enzymes
The protein content of honey is roughly ranged from 0.2-0.5% in forms of enzymes and free amino acids. Generally, the total amount of free amino acid in honey range approximately between 10 and 200 mg/100 g honey and proline contribute with 50% of total amino acid [].G-aminobutyric acid and ornithine have been identified in honey samples in addition to b-alanine and a-alanine [].
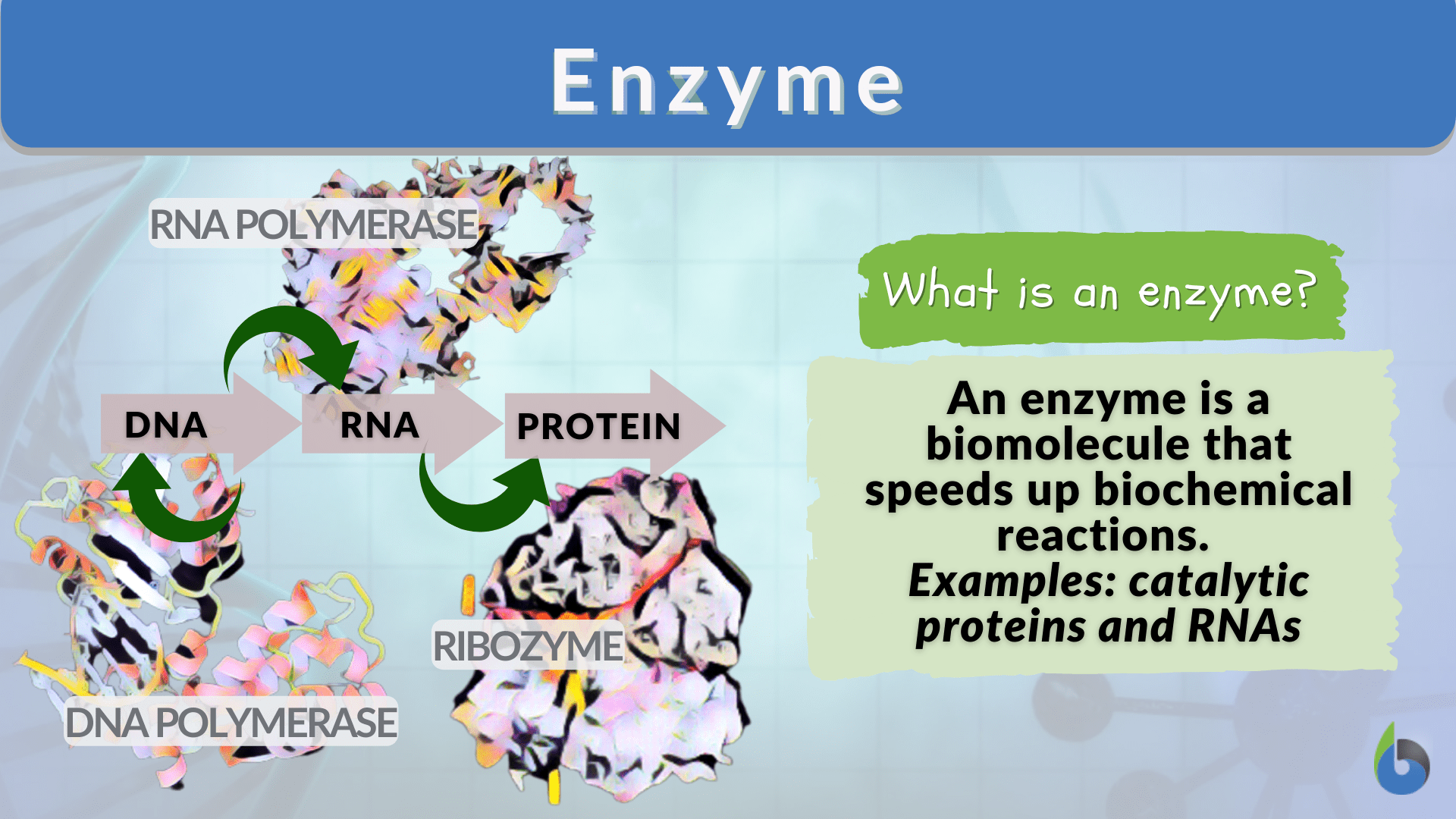
Enzyme Definition and Examples Biology Online Dictionary
Apparently there is something going around about how metal will break down the beneficial enzymes in honey. I did a little looking around and it's technically possible, since honey is acidic, but not with stainless, and even with other metals the reaction would take WAY longer than the amount of time honey is on a spoon.
4 Important Properties Of Enzymes Infinita Biotech
However, we do not recommend storing a metal spoon within your honey for long periods of time. 7. Honey can be used on wounds Fact. Up until the early 20 th century, honey was used as a conventional therapy in fighting infection. 8. All bees produce honey Myth. There are nearly 20,000 known bee species in the world. From this number, only 5%.
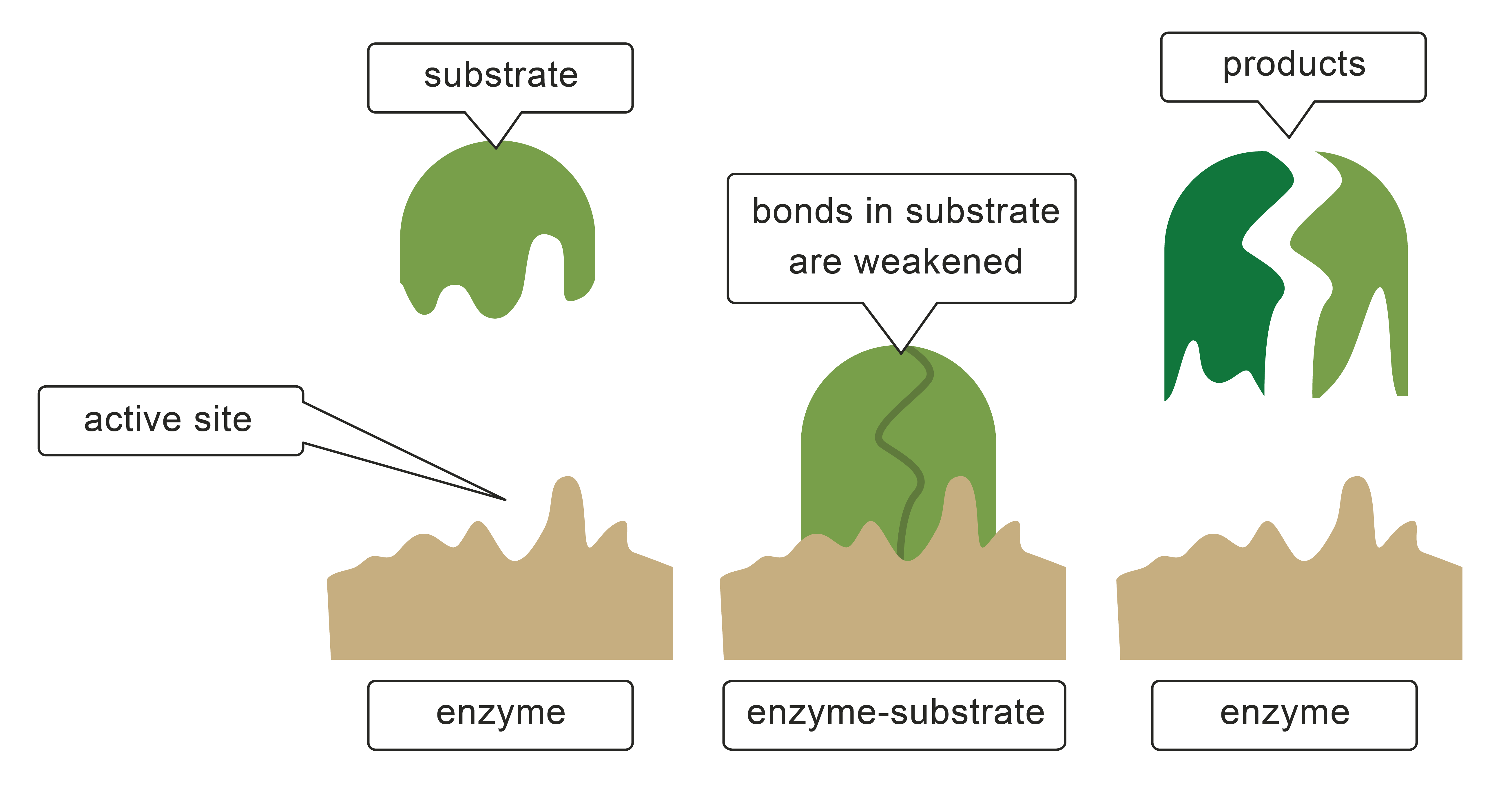
Enzymes I The Unsung Heroes of the Body The Essential Way
In conclusion, using a metal spoon to scoop raw honey may not necessarily kill enzymes in honey. However, it is important to note that honey is acidic due to its organic acid content, and the pH scale of honey is normally between 3.4 to 6.1. Acidic substances can corrode metals, and it is often feared that metal components can be mixed in honey.