
SOLVED Part € Rank the following from most to least acidic Rank from
Hello, my name is john, and for this question we have to rate the following pieces of a p h, values to the least ascetic to most ascetic. So the first thing that we're going to do is convert everything into the ph values so from the given amounts from left to right, we have ph of 4, and the concentration of h, 3 plus is going to be a ph of 5, the next 1.

Chemistry Archive April 19, 2017
This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Question: Rank the following from most acidic to least acidic, Rank these items from most acidic to least acidic. To rank items as equivalent, overlap them. View Available Hint (s) Res pH-5 pH-3 pH 14 H301 - 10 [H:01 - 10.
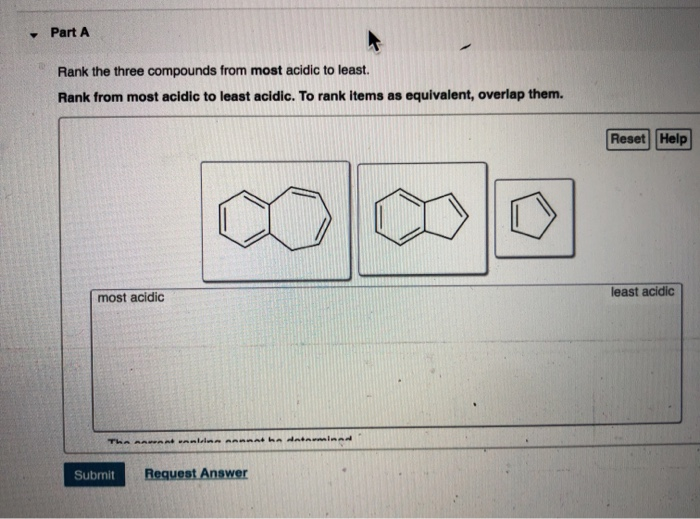
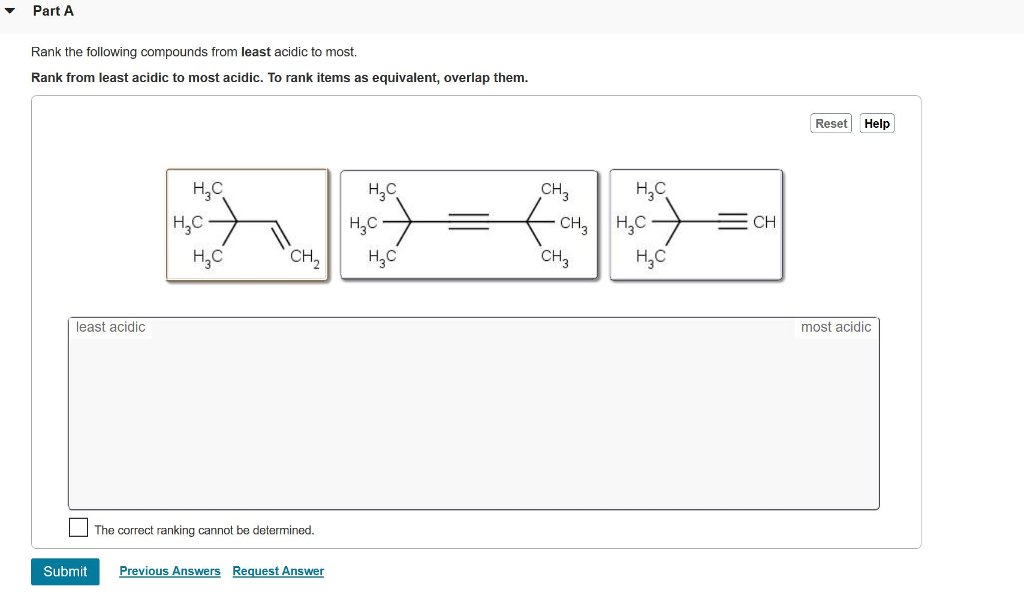
Solved Part A Rank the three compounds from most acidic to
Rank these compounds from the least acidic to the most acidic. Skip to main content. Organic Chemistry Start typing, then use the up and down arrows to select an option from the list.. Rank these compounds from the least acidic to the most acidic. ANSWERS OPTIONS. A. I II III. B. III I II. C. III II I. D. I III II. Show Answer. Previous.
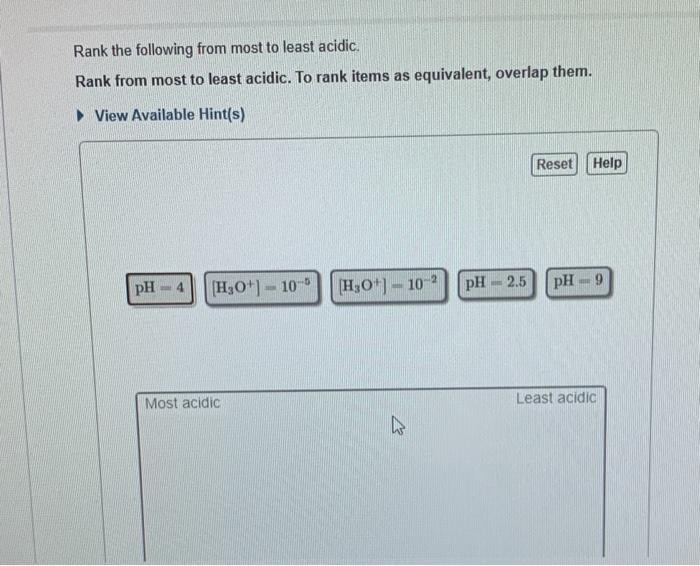
Solved Rank the following from most to least acidic. Rank
Rank these from least acidic to highest acidic. H_3PO_4, CH_3COOH, CH_3OH, CHCl_2CH_2CH_2OH. Order from most acidic to least acidic: - CH_3CH_2OH - CH_3CH_2COOH - CH_3CHClCOOH - ClCH_2CH_2COOH; Order the hydrogens in the following molecule by acidity (from least acidic to most acidic). Rank the given compounds based on their relative acidities.
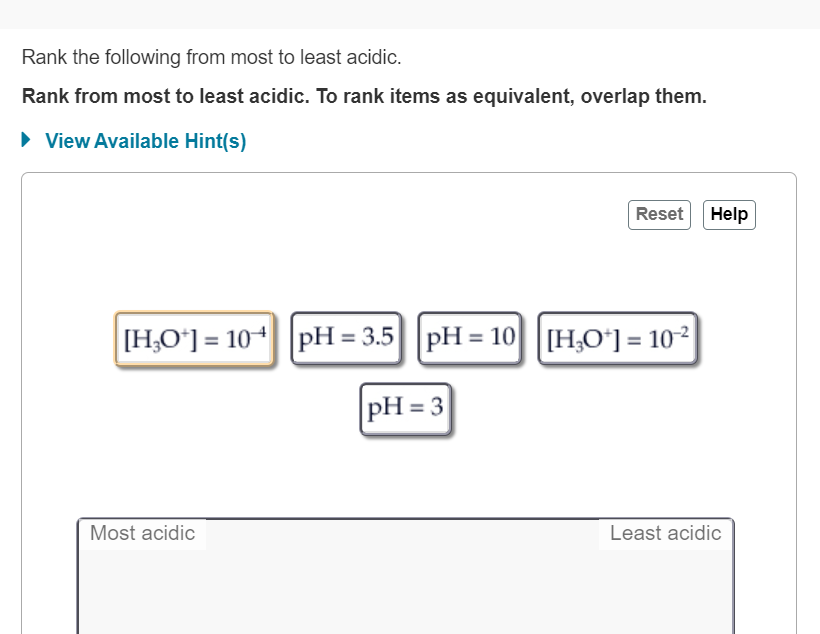
Solved Rank the following from most to least acidic. Rank
Rank these items from most acidic to least acidic. To rank items as equivalent, overlap them. and more. Study with Quizlet and memorize flashcards containing terms like What is the pH of an aqueous solution with the hydronium ion concentration [H3O+] = 2 x 10-14 M ? Make sure that your answer has the correct number of significant figures.
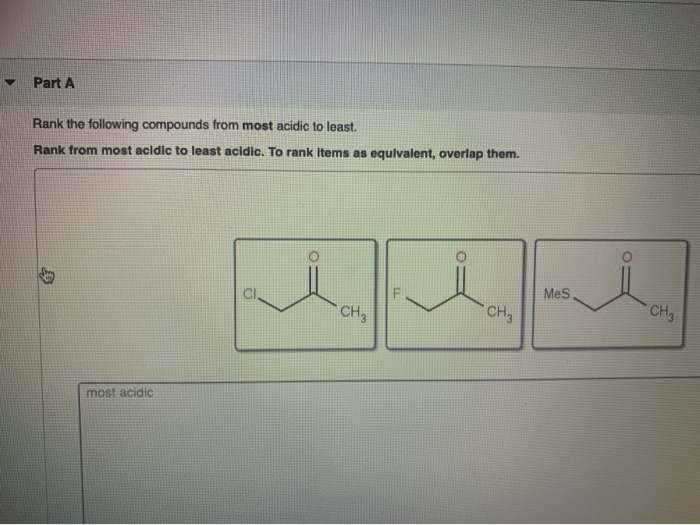
Solved Part A Rank the following compounds from most acidic
Rank the following from most acidic to least acidic. Rank these items from most acidic to least acidic. To rank items as equivalent, overlap them. View Available Hint(s) Reset Hellp pH=3 pH=5 [H_3O^+]=10^(-4) [H_2O^+]=10^(-2) pH=14 least acidic most acidic
Solved Rank the following from most to least acidic Rank
Rank the following phenols based on acidity (1 = most acidic and 6 = least acidic). Rank the following compounds from most acidic to least. To rank items as equivalent, overlap. Rank the following molecules 1-4 based on the rank in decreasing acidity. (1 = least acidic, 4 = most acidic). Rank the following phenols from most to least acidic.
Solved Part A Rank the following compounds from least acidic
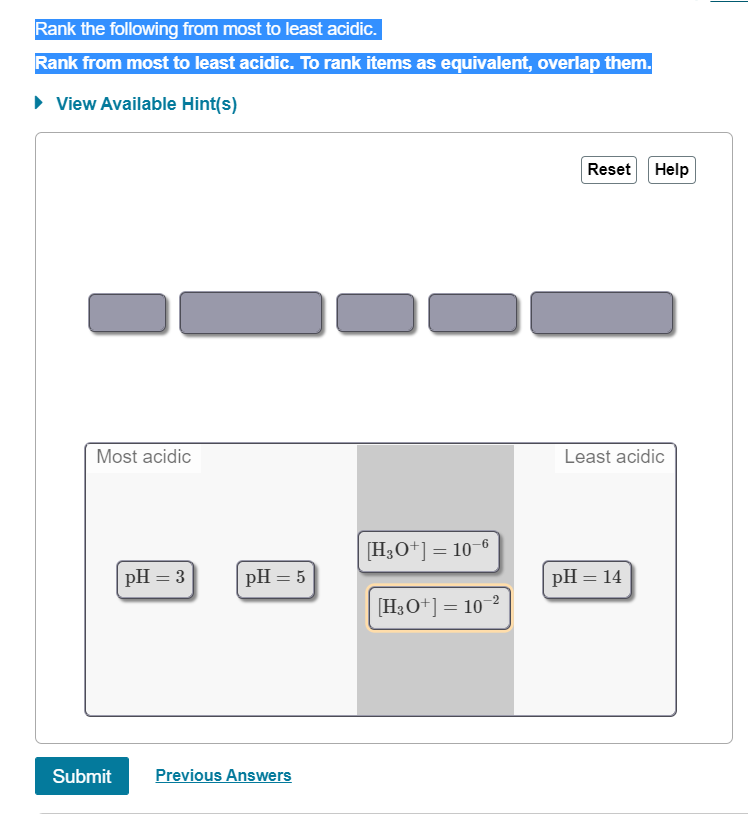
Rank the following from most to least acidic Rank from most to least acidic. To rank items as equivalent; overlap them_ View Available Hint(s) Reset Hel [Hzo+] 10 pH 2.5 pH pH [Hz0'] 10 Most acidic Least acidic
Rank the following compounds from most acidic (rank 1… SolvedLib
Rank these items from most acidic to least acidic. To rank items as equivalent, overlap them. The 10-6 M H3O+ solution has a pH of 6, and the 10-2 M H3O+ solution has a pH of 2. The solutions are ranked from most acidic (pH = 2) to most basic (pH = 14). CO2 in ocean.
Solved Rank the following from most to least acidic. Rank
VIDEO ANSWER: There are two things that you need to know to solve this problem. The first thing is that low p h means more acitic, and the second is that p h equals the negative of the log base 10 of the concentration of h, 3. O plus means that if h
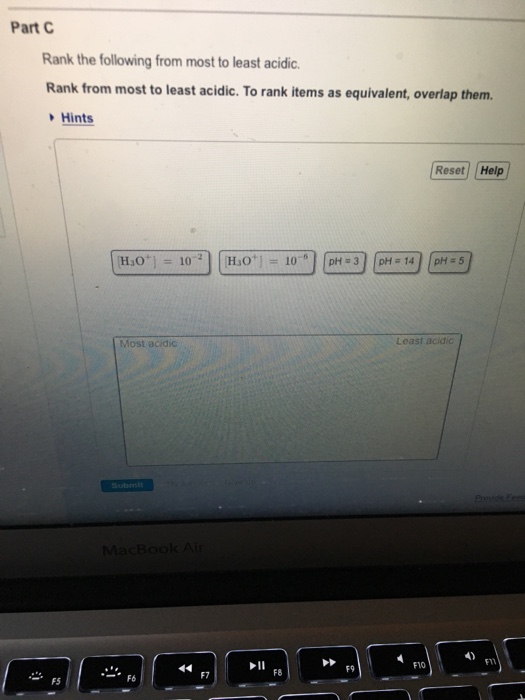
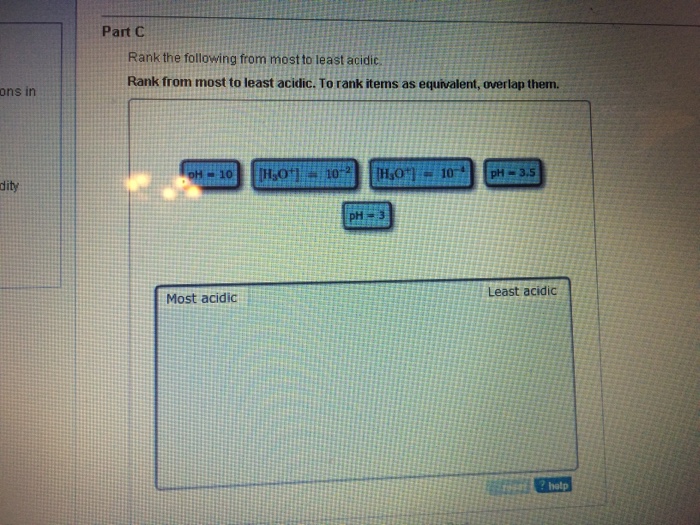
Solved Part C Rank the following from most to least acidic
Rank the following trom most acidic to least acidic, Rank these items Irom most acidic least acidic; To rank items as equivalent, overlap them: View Available Hint(s) (,o" [H,o' 01:01. Rank the following compounds from strongest acid to weakest acid: 02:30. Rank the following compounds in order of decreasing acidity:

Solved most acidic, and 4 = least acidic 5. a) Rank these
Rank these items from most acidic to least acidic. [H3O+]=10−2. pH = 3. pH = 5. [H3O+]=10−6. pH = 14. - The 10-6 M H3O+ solution has a pH of 6, and the 10-2 M H3O+ solution has a pH of 2. The solutions are ranked from most acidic (pH = 2) to most basic (pH = 14). Study with Quizlet and memorize flashcards containing terms like What is the.
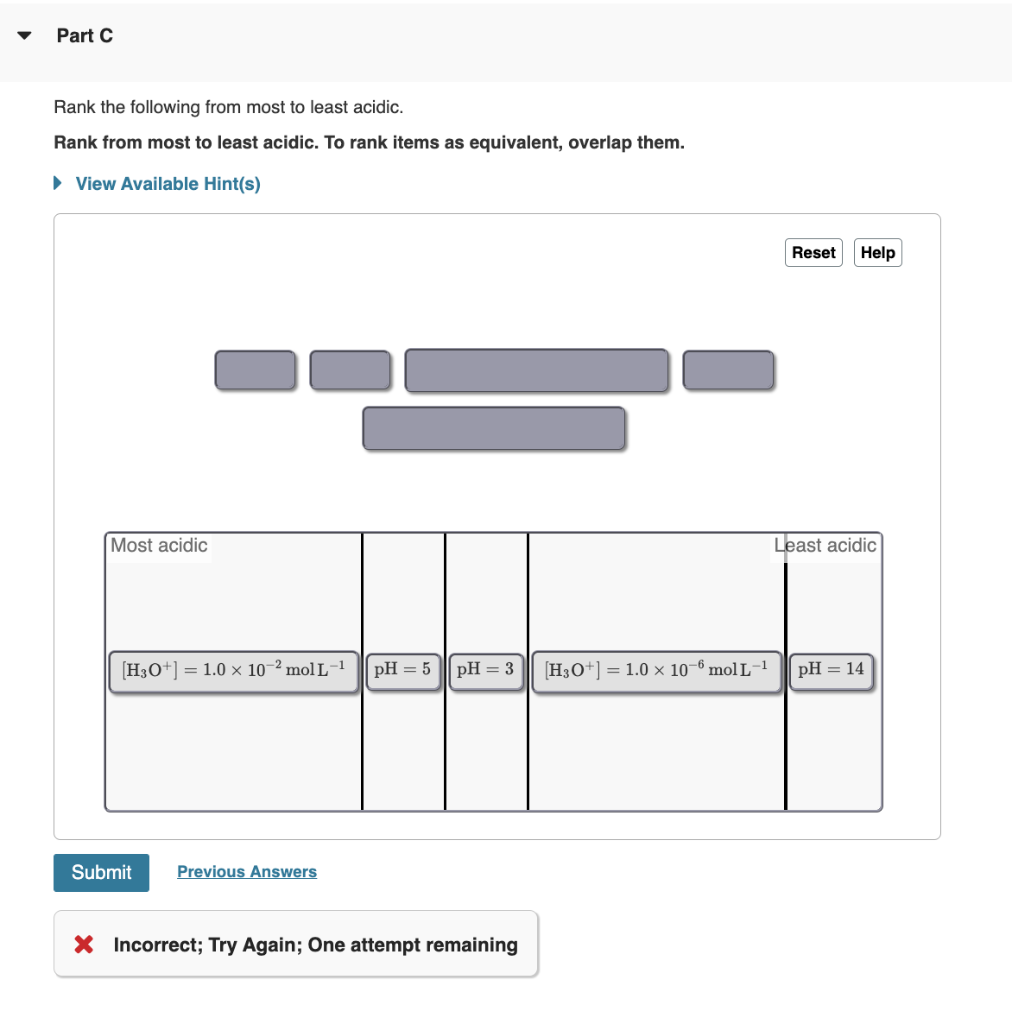
Solved Rank the following from most to least acidic. Rank
1. 12 PRACTICE PROBLEM. Compare and arrange these compounds in increasing order of acidity. Provide a suitable reason for your answer. CH 3 CH 2 OH, HCOOH, HCl, HBr, CH 4, and CH 3 CH (Cl)COOH. 13 PRACTICE PROBLEM. Explain why FCH 2 COOH is more acidic than ICH 2 COOH if HF is less acidic than HI.
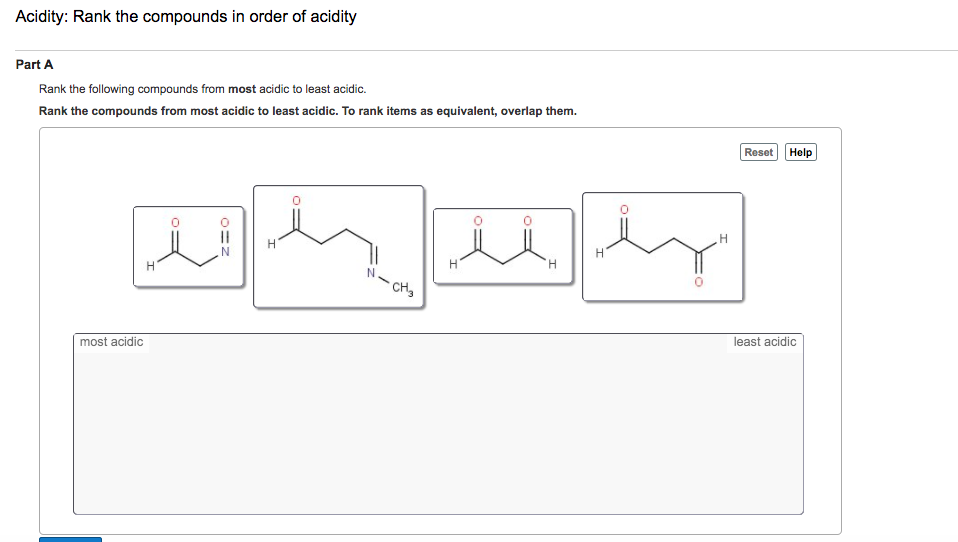
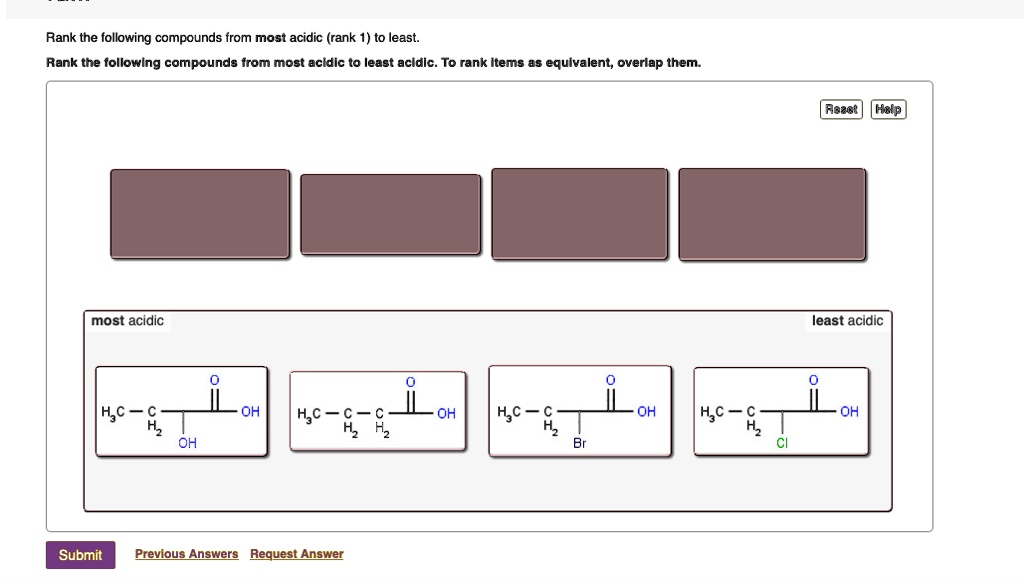
Solved Rank the following compounds from most acidic to
Because fluoride is the least stable (most basic) of the halide conjugate bases, HF is the least acidic of the haloacids, only slightly stronger than acetic acid. HI, with a pK a of about -9, is one the strongest acids known. More importantly to the study of biological organic chemistry, this trend tells us that thiols are more acidic than.
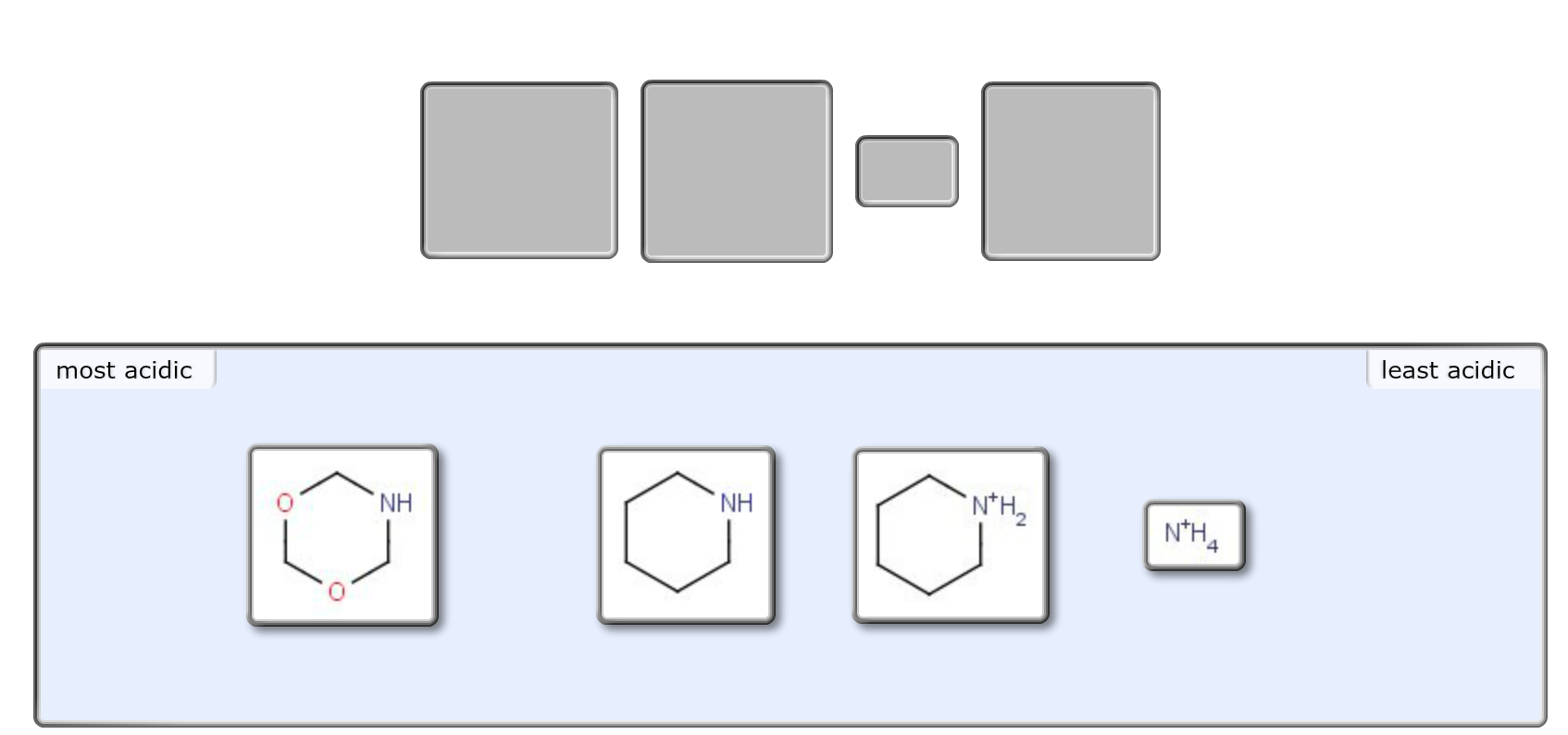
Solved Rank the following compounds from most acidic to
Rank these items from most acidic to least acidic. To rank items as equivalent, overlap them. View Available Hint(s) Rese HO 10 HO) - 10 pH 14 pH 5 pH 3 Thapter 3 hapter 3 Question 12 match the terms in the sent coumn to the appropriate banks in the sentences on the night. Terms can be used once, more than once, or nor at an < 14 of Reset Help.
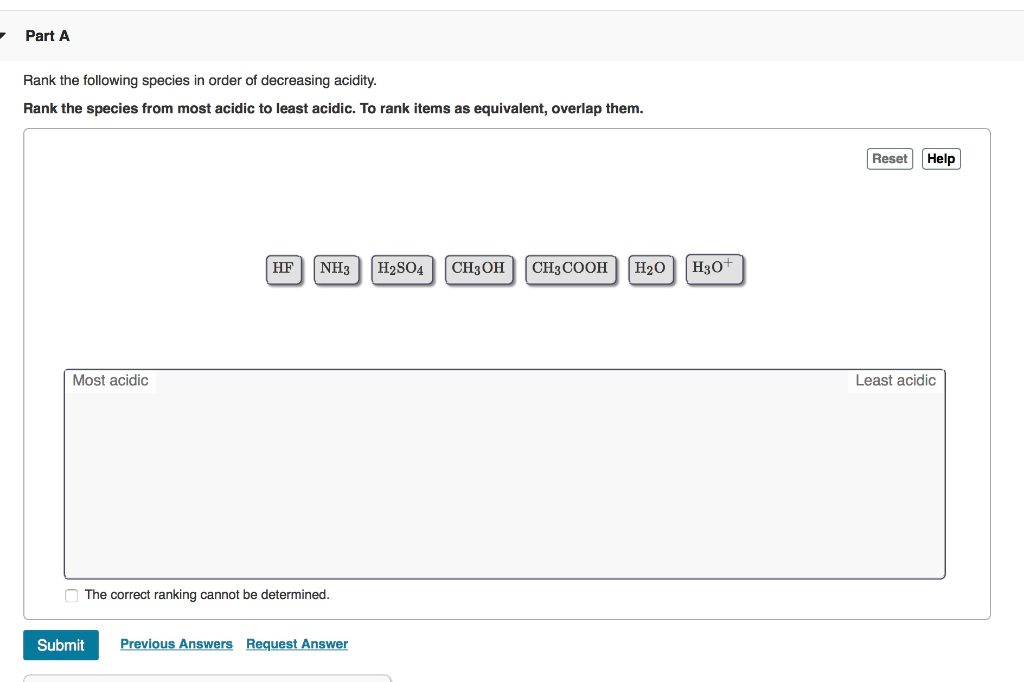
Solved Rank the following species in order of decreasing
Ranking of the compounds from most acidic to least acidic: H₂SO4; NH4NO3; NaCN; NaOH; NaCl; Acidity is determined by the presence of hydrogen ions (H+). Compounds that release more hydrogen ions are more acidic. In this case, H₂SO4 is the most acidic because it is a strong acid that completely dissociates in water, releasing two hydrogen ions.