These Daily Uses of pblock Elements will Surprise You askIITians
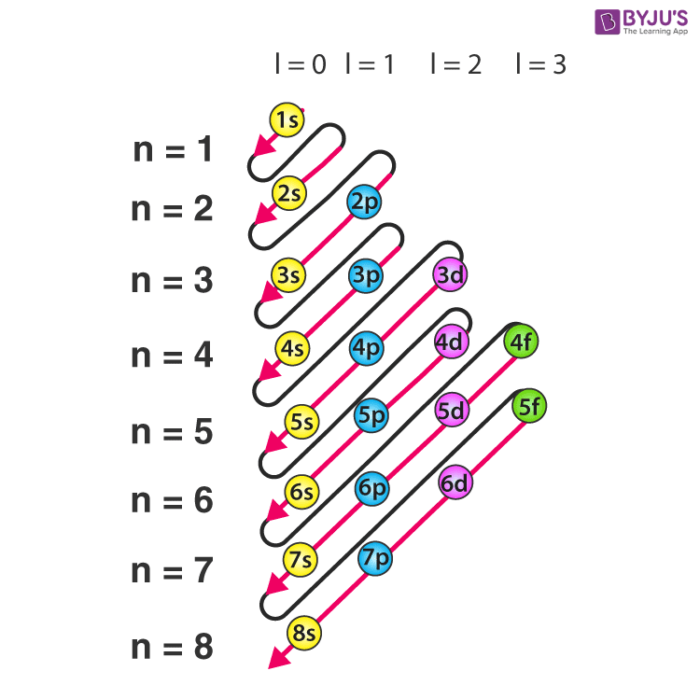
This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.
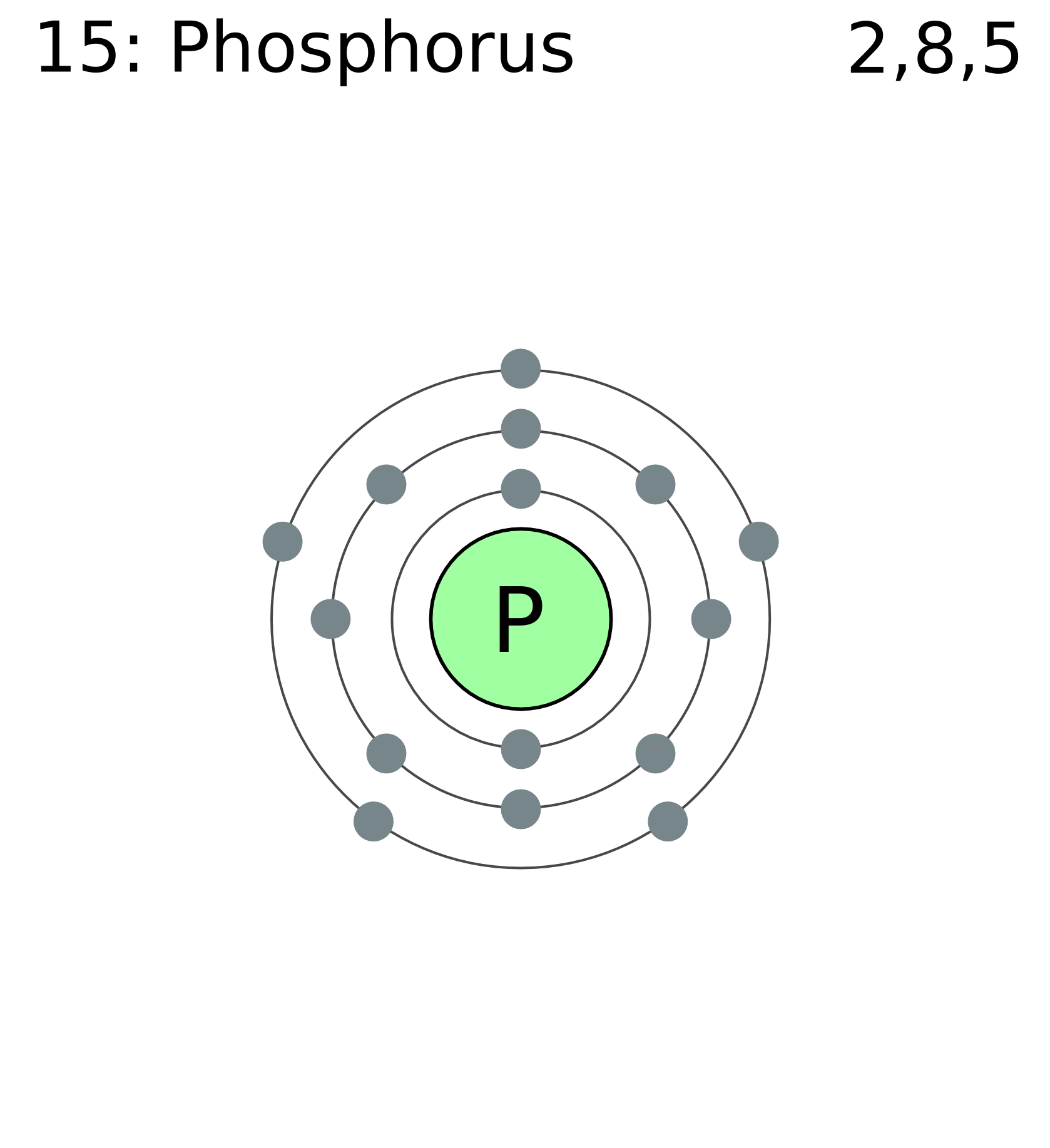
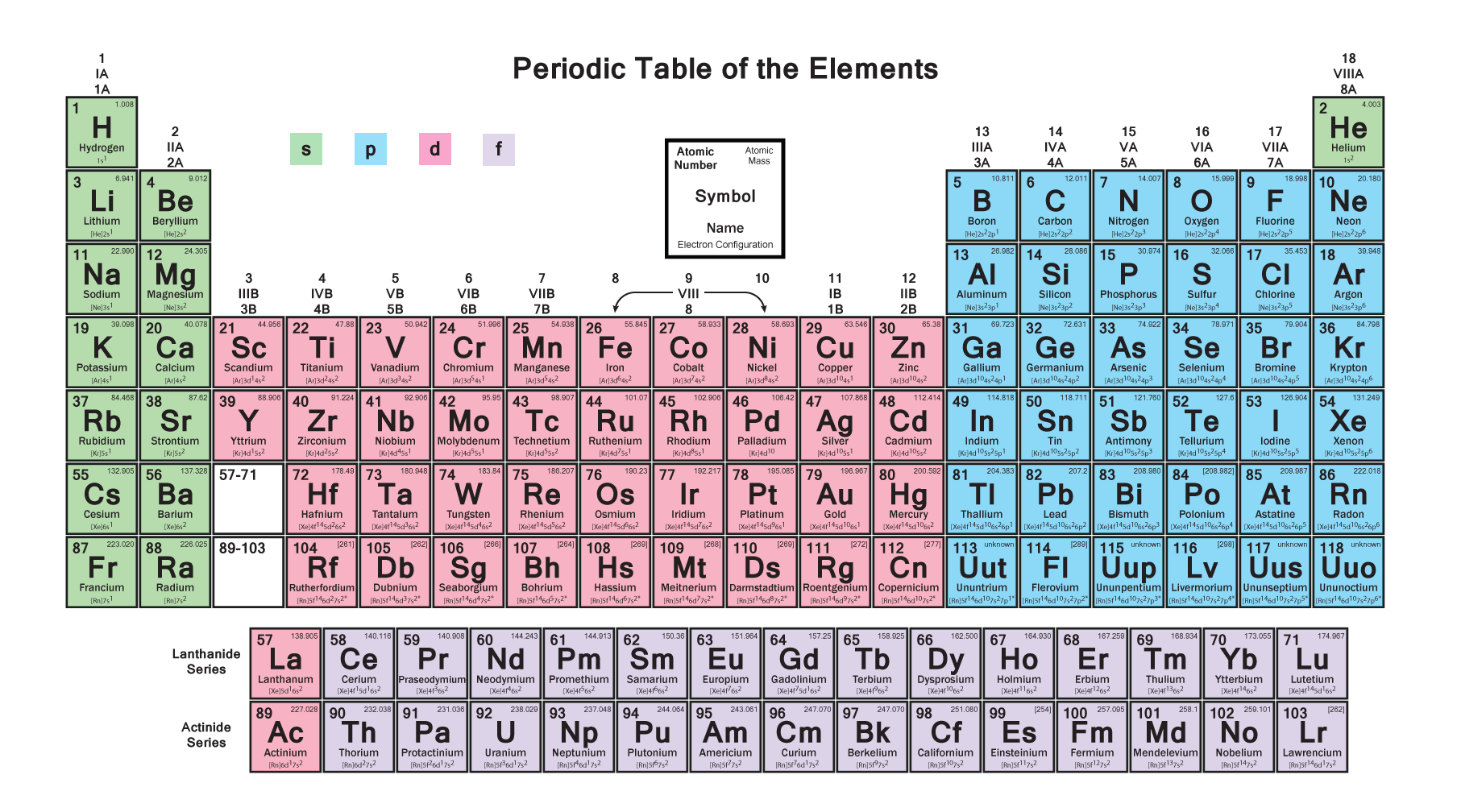
Phosphorus Electron Configuration (P) with Orbital Diagram
1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2). You will get the detailed information about the periodic table which will convert a newbie into pro. 3). You will also get the HD images of the Periodic table (for FREE).

Electronic Configuration How To Write Electron ConfigurationChemistry
Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. - helps chemist understanding how elements form chemical bonds. - can be written using the period table or an electron configuration chart. How to Write the Electron Configuration for Phosphorus (P)
Electron Structure ALevel Chemistry Revision Notes
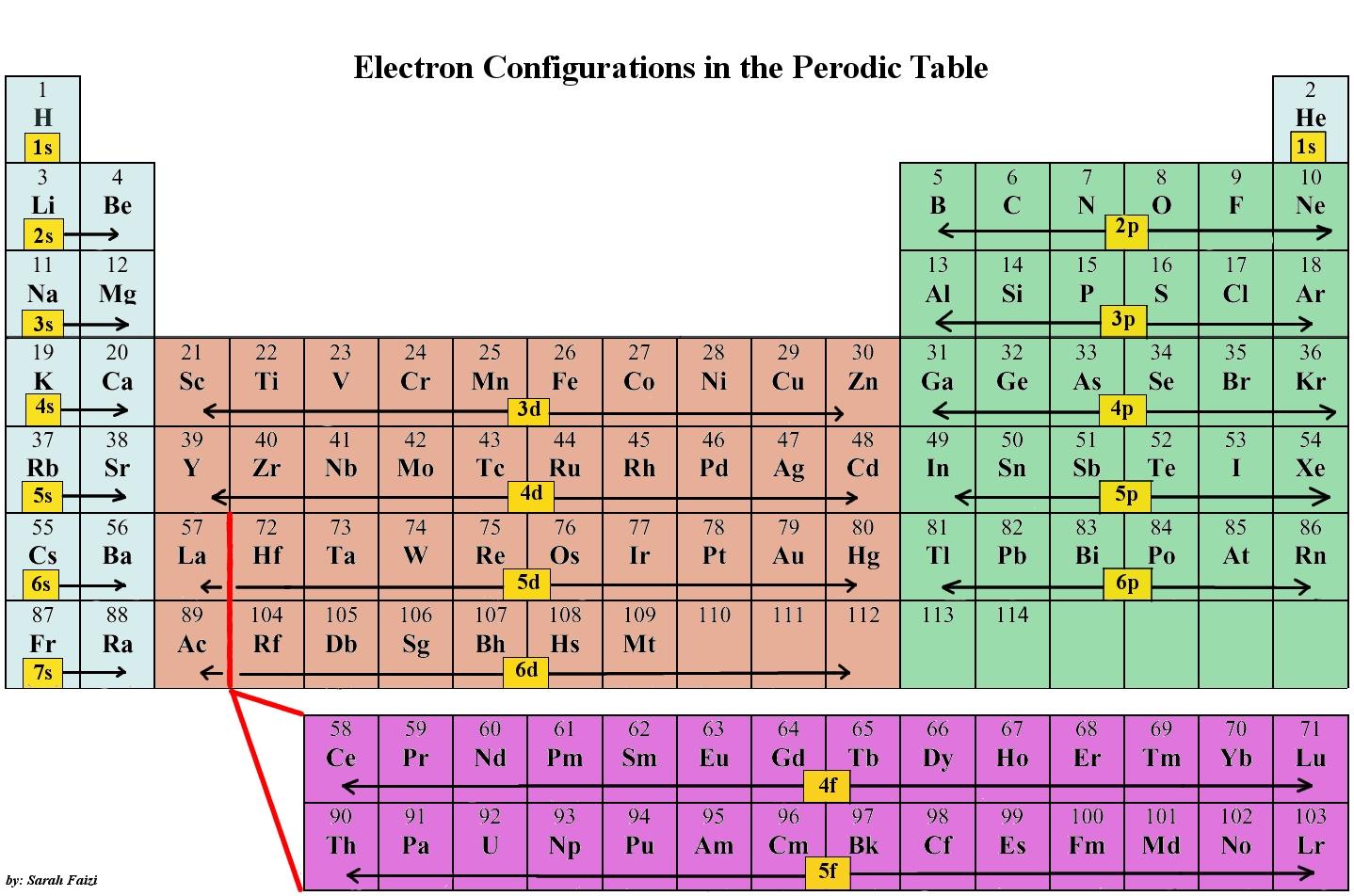
Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. The electron configuration for the first 10 elements. H #1s^1# He #1s^2# Li #1s^2 2s^1# Be #1s^2 2s^2# B #1s^2 2s^2 2p^1#

Write the general outer electronic configuration of `s`,`p`,`d` and
Electron configurations are a simple way of writing down the locations of all of the electrons in an atom. As we know, the positively-charged protons in the nucleus of an atom tend to attract negatively-charged electrons.
Electronic configuration detailed explanation, orbital filling
This electron configuration is written as 1 s2 2 s1. The next element is beryllium, with Z = 4 and four electrons. We fill both the 1 s and 2 s orbitals to achieve a 1 s2 2 s2 electron configuration: When we reach boron, with Z = 5 and five electrons, we must place the fifth electron in one of the 2 p orbitals.

Electron configuration periodic table qustbh
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms= + 1 2 1 2 ).
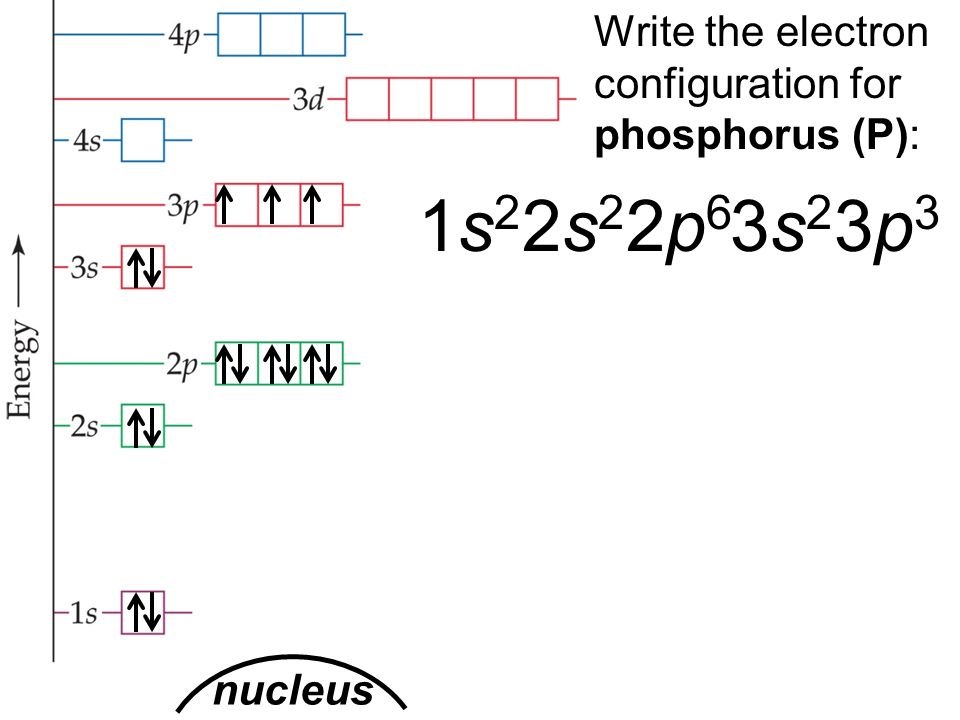
Phosphorus Electron Configuration (P) with Orbital Diagram
The arrangement of electrons in phosphorus in specific rules in different orbits and orbitals is called the electron configuration of phosphorus. The electron configuration of phosphorus is [ Ne] 3s 2 3p 3 , if the electron arrangement is through orbitals. Electron configuration can be done in two ways.

Electronic Configuration Of Elements Trick (s,p,d,f) Pattern Class11
The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number between 0 and 14. Thus in the building-up process for the lanthanoids.

P and P 3 Electron Configuration (Phosphorous and Phosphide Ion) YouTube
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure 6.25 ): The number of the principal quantum shell, n,
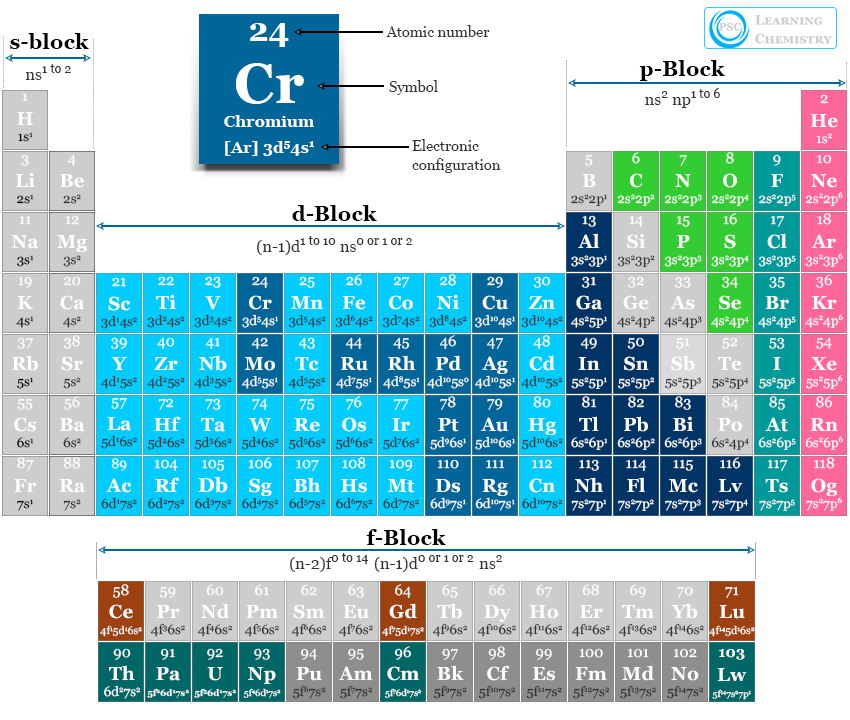
Electron Configuration Periodic Table Elements Chemistry
The electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s orbital and finally the d orbitals (if any more electrons need to be removed). For instance, the ground state electronic configuration of calcium (Z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2.

6.9 Elektronové konfigurace a periodická tabulka Chemistry
In this video we will write the electron configuration for Phosphours (P) and P 3- (the Phosphide ion). We'll also look at why Phosphorous forms a 3- ion and.
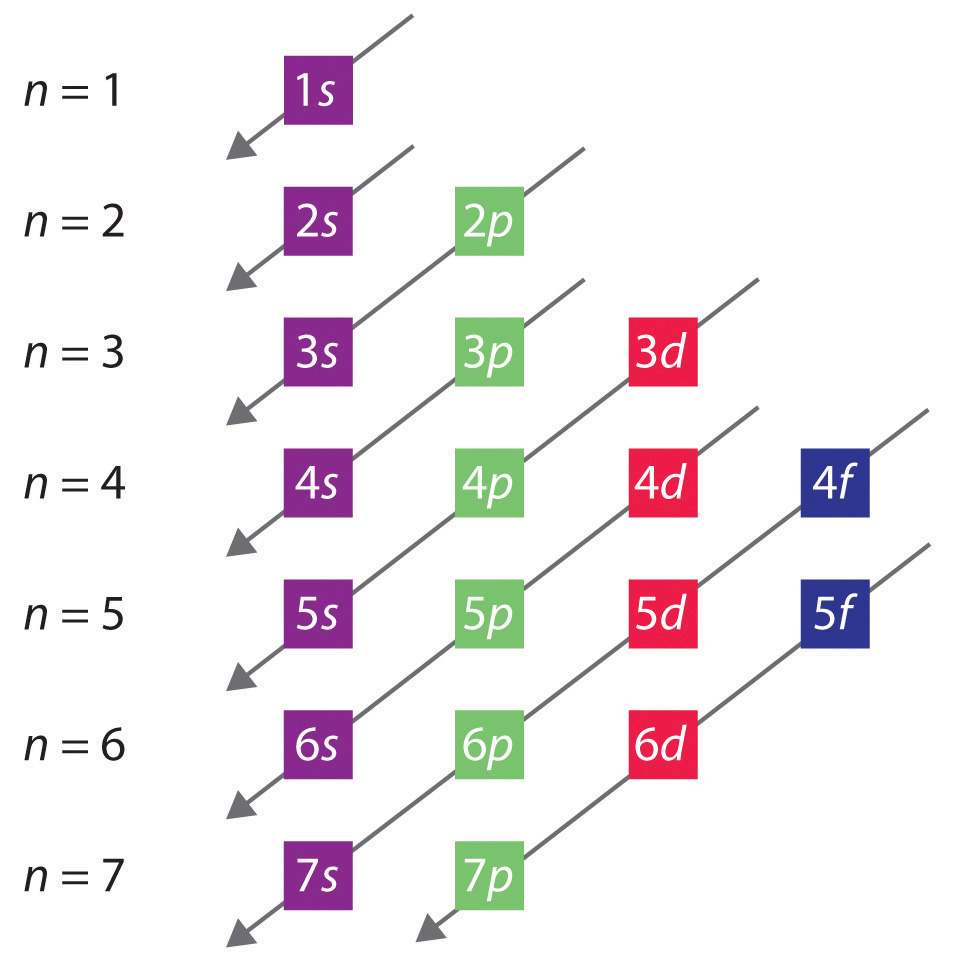
Question 9267e Socratic
In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic table gives the following electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3.
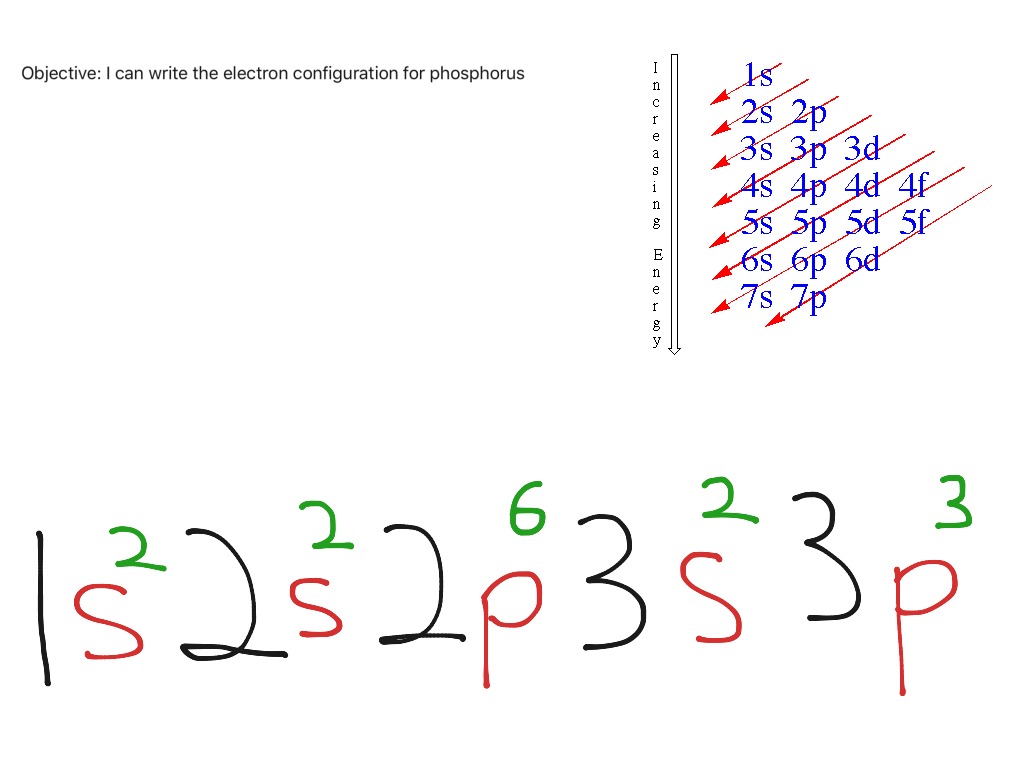
Electron configuration example for phosphorus Science, Chemistry
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure 3.1.2 3.1. 2 ): The number of the principal quantum shell, n, The letter that designates the orbital type (the subshell, l ), and

Electronic Configuration of a Atom Atomic Structure Electronic
0:00 / 1:45 A step-by-step description of how to write the electron configuration for Phosphorus (P). In order to write the P electron configuration we first need to kn.
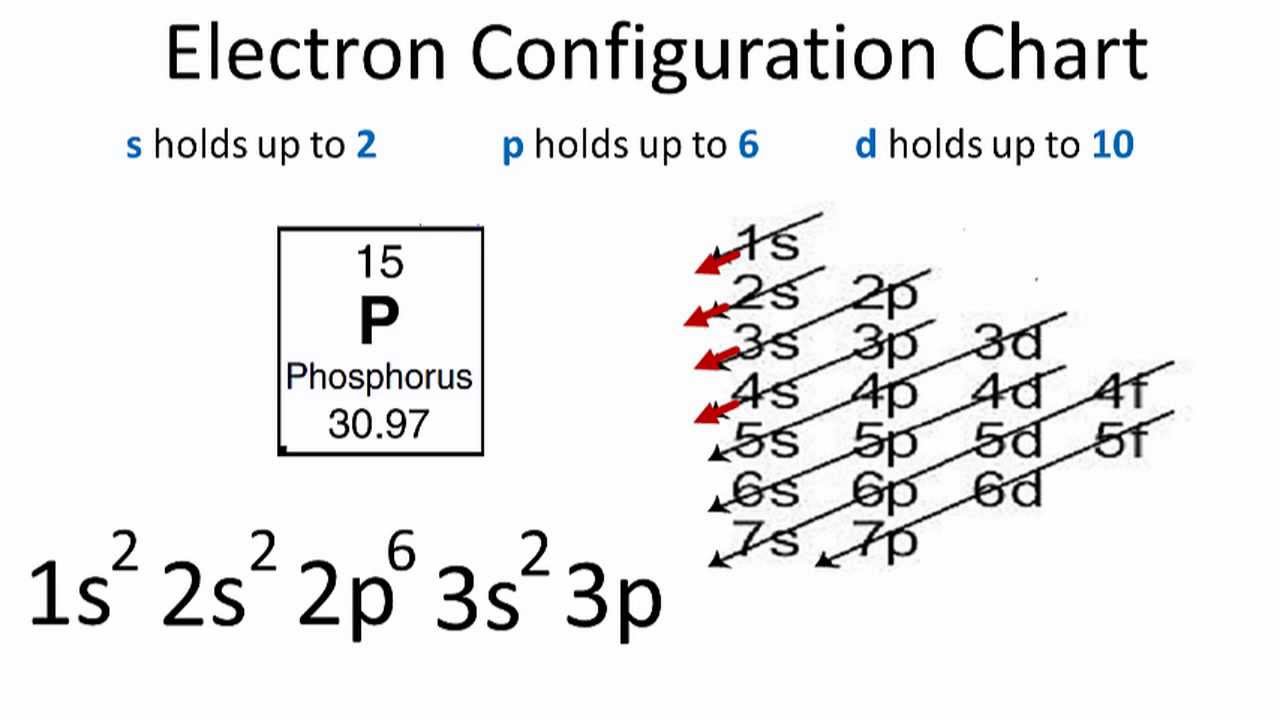
Phosphorus Electron Configuration (P) with Orbital Diagram
AboutTranscript. Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Created by Sal Khan.