Bohr Diagram The Element Sulfur
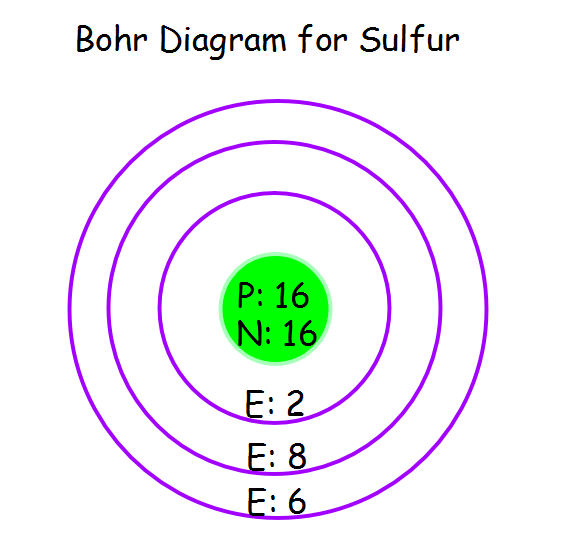
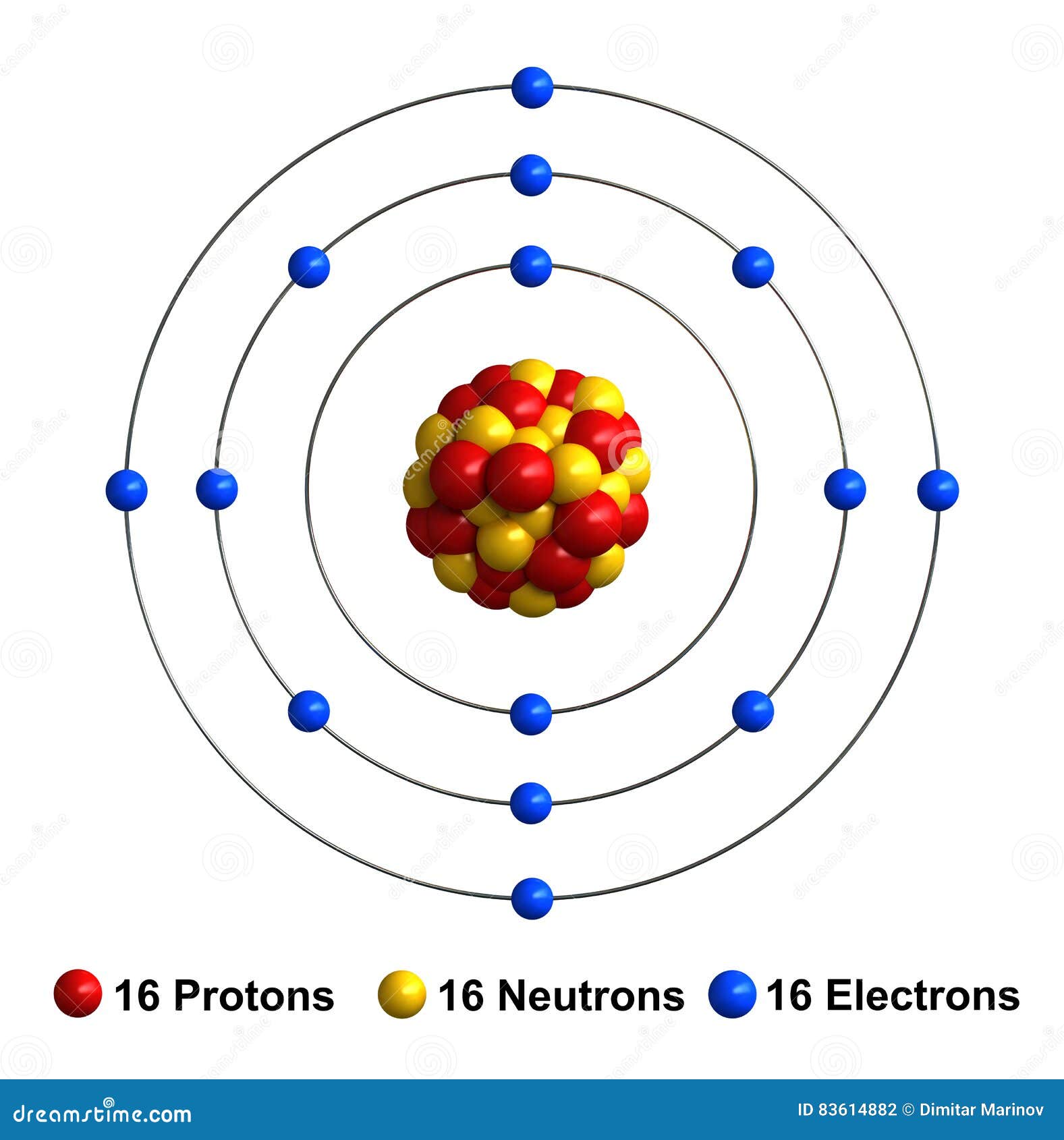
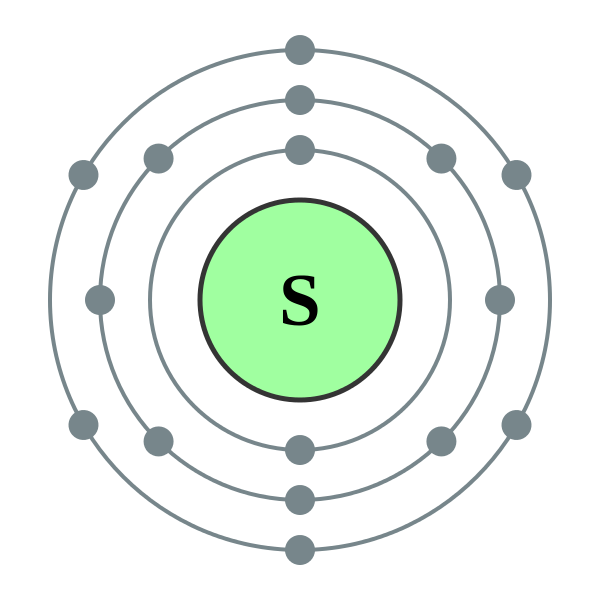
The sulfur atom has 16 protons, 16 neutrons and 16 electrons in three different energy levels, or orbits. Physics suggests that electrons do not physically exist as "points," but teachers use the Bohr atom model with fixed electrons as a way to simplify atomic structure. Creating the model requires the ability to cut with scissors and use glue.
Bohr Diagram Of Sulfur
0:00 / 2:45 How to Draw the Bohr-Rutherford Diagram of Sulfur chemistNATE 247K subscribers Subscribe 415 32K views 4 years ago Sulfur / Sulphur has 2 electrons in its first shell, 8 in its.

Bohr Diagram Of Sulfur Wiring Diagram
For example, sulfur has an atomic number of 16, so its Bohr diagram would show two electrons in the first energy level and eight electrons in the second energy level. The Bohr diagram provides a visual representation of an element's electron arrangement, making it easier to understand and analyze the behavior of atoms.

Sulfur Bohr Model — Diagram, Steps to Draw Techiescientist
March 23, 2023 by Jay Bohr model of all Elements is mentioned in the chart below. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. 1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2).
Sulfur (S) AMERICAN ELEMENTS
On the far left of Figure 3.6.1 3.6. 1 are the highest energy electromagnetic waves. These are called gamma rays and can be quite dangerous, in large numbers, to living systems. The next lower energy form of electromagnetic waves are called x-rays. Most of you are familiar with the penetration abilities of these waves.
Sulfur Stock Illustrations 1,549 Sulfur Stock Illustrations, Vectors
The Bohr model of the hydrogen atom (Z = 1) or a hydrogen-like ion (Z > 1), where the negatively charged electron confined to an atomic shell encircles a small, positively charged atomic nucleus and where an electron jumps between orbits, is accompanied by an emitted or absorbed amount of electromagnetic energy (hν). The orbits in which the electron may travel are shown as grey circles; their.

Bohr Diagram Of Sulfur Wiring Diagram
Bohr model of calcium: (CC BY-SA 2.0 uk;Greg Robson): Answer b. Bohr model of sulfur: (CC BY-SA 2.0 uk; Greg Robson). Valence electrons are located in the highest energy level of an atom. When drawing a Bohr diagram, the valence electrons would be present in the outermost electronic level/shell (furthest away from the nucleus).

Bohr Diagram Of Sulfur Wiring Diagram
Sulfur Bohr Model — Diagram, Steps to Draw Sulfur has the atomic number 16 and is denoted by the chemical symbol S. Under normal conditions, sulfur occurs as a cyclic octatomic molecule represented by formula S 8.
Bohr Diagram Of Sulfur
In this video we'll look at the atomic structure and Bohr model for the Sulfur atom (S). We'll use a Bohr diagram to visually represent where the electrons a.
Bohr Diagram For Sulfur
Now let's draw the Bohr orbital diagram of sulfur. 16 protons and 16 neutrons are located in the nucleus which is surrounded by 16 electrons: Sulfur has 2 electrons in the first shell, 8 electrons in the second shell, and 6 electrons in the third shell. Create an account to view solutions.
35 Bohr Diagram Of Sulfur Wiring Diagram List
The Bohr Rutherford diagram is a model that shows the arrangement of electrons within an atom. It was developed by Niels Bohr and Ernest Rutherford to explain the behavior of electrons in an atom. In the Bohr Rutherford diagram of sulfur, the nucleus is represented by a small dot in the center.

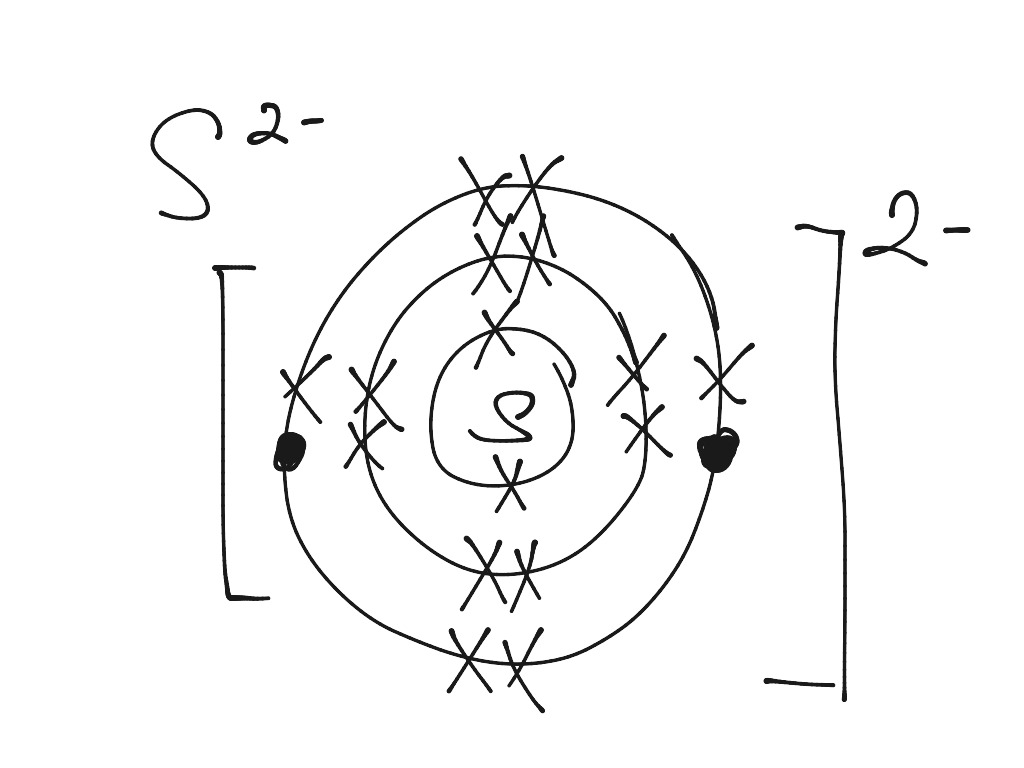
modified Bohr diagram for an atom of sulfur34. Did I do it correctly
Bohr diagram is very interesting and easy to draw. Here, we will draw the Bohr diagram of the Sulfur atom with some simple steps. Steps to draw the Bohr Model of Sulfur atom 1. Find the number of protons, electrons, and neutrons in the Sulfur atom
11+ Sulfur Bohr Diagram Robhosking Diagram
Bohr diagrams of various elements. Image credit: OpenStax Biology. Electron configurations and the periodic table. Elements are placed in order on the periodic table based on their atomic number, how many protons they have. In a neutral atom, the number of electrons will equal the number of protons, so we can easily determine electron number.

Bohr Diagram Of Sulfur
Physical & Theoretical Chemistry Supplemental Modules (Physical and Theoretical Chemistry) Electronic Structure of Atoms and Molecules

Bohr Diagram Of Sulfur
Electron configuration through orbit (Bohr principle) Electron configuration through orbital (Aufbau principle) Sulfur (S) atom electron configuration (Bohr model) Electron configuration through orbitals follows different principles. For example Aufbau principle, Hund's principle, and Pauli's exclusion principle.

Diagram representation of the element sulfur Vector Image
The Bohr Model is a modification of an earlier atomic model, the Rutherford Model. The Bohr Model has an atom with a positively-charged nucleus surrounded by negatively-charged electrons that have circular, planetary-like orbits. Today, we know that the Bohr Model has some inaccuracies, but it's still used because of its simple approach to.