
SOCl2 Lewis Structure Thionyl chloride YouTube
It is possible to draw a structure with a double bond between a boron atom and a fluorine atom in BF 3, satisfying the octet rule, but experimental evidence indicates the bond lengths are closer to that expected for B-F single bonds. This suggests the best Lewis structure has three B-F single bonds and an electron deficient boron.
[Solved] draw lewis structure and molecular geometry of xeo4 SO2CL2
A quick explanation of the molecular geometry of SOCl2 including a description of the SOCl2 bond angles.Looking at the SOCl2 Lewis structure we can see that.
So2cl2 Molecular Geometry
The Lewis structure for SOCl 2 requires you to place more than 8 valence electrons on Sulfur (S). You might think you've got the correct Lewis structure for SOCl 2 at first. Remember, Sulfur is in Period 3 and can hold more than 8 valence electrons. You'll want to calculate the formal charges on each atom to make sure you have the best Lewis.

H2S Lewis StructureThis post is about the lewis structure of hydrogen
Sulfor dioxide: Lewis dot structure for SO2 (video) | Khan Academy Chemistry library Course: Chemistry library > Unit 9 Lesson 4: Dot structures and molecular geometry Resonance and dot structures Formal charge Formal charge and dot structures Worked example: Using formal charges to evaluate nonequivalent resonance structures
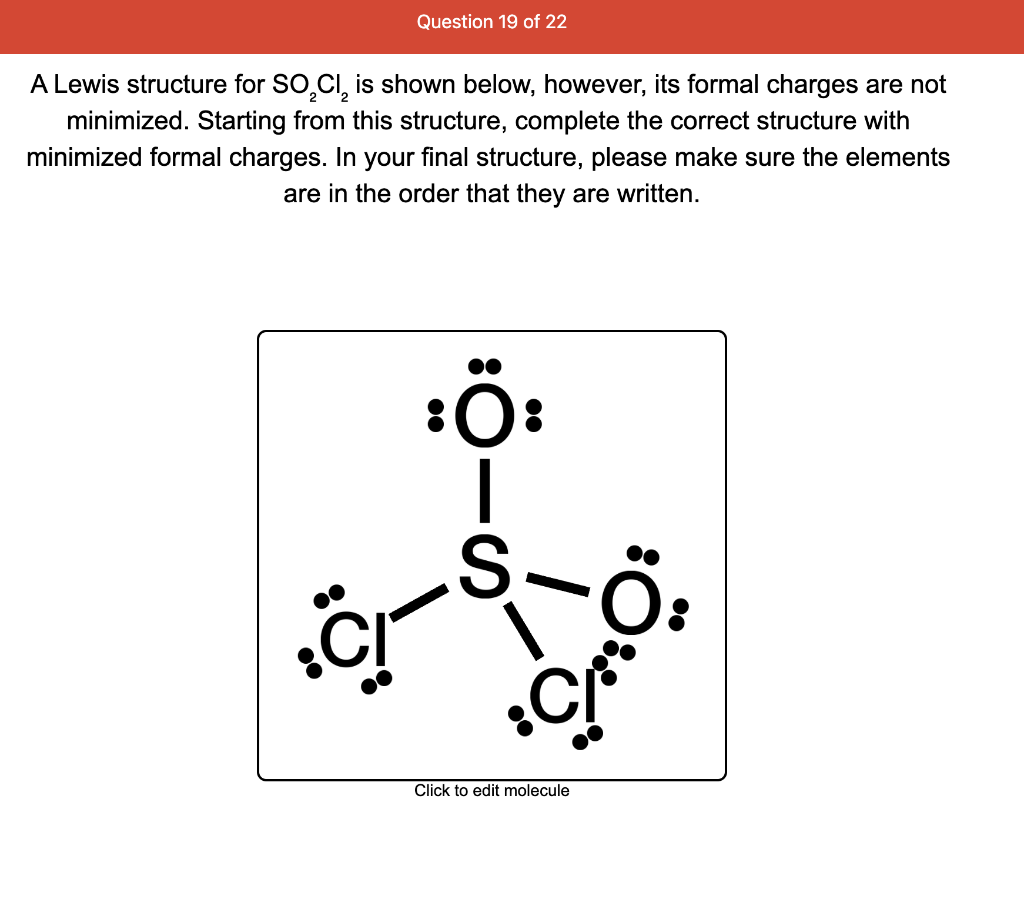
Solved A Lewis structure for SO2Cl2 is shown below, however,
In the SO2Cl2 lewis structure, the whole electron density lies around the central S atom, and two Cl and two O are present at four sites of the tetrahedral moiety. The ideal bond angle should be 109.5 0 for tetrahedral but here the scenario is different.

So2Cl2 Lewis Structure
SO2Cl2 lewis structure has a Sulfur atom (S) at the center which is surrounded by two Oxygen atoms (O) and two Chlorine atoms (Cl). There are single bonds between the Sulfur & Chlorine atoms and double bonds between the Sulfur & Oxygen atoms. There are 2 lone pairs on Oxygen atoms (O) and 3 lone pairs on Chlorine atoms (Cl).

draw the lewis structure of SOCl2 Brainly.in
In the SO 2 Cl 2 Lewis structure, there are two single bonds and two double bonds around the sulfur atom, with two chlorine atoms and two oxygen atoms attached to it. Each chlorine atom has three lone pairs, and each oxygen atom has two lone pairs. Lewis dot structure for SO2Cl2 Sulfuryl chloride Watch on Contents Steps #1 First draw a rough sketch

Draw the Lewis structure for SO2Cl2 and then answer questions that
Lewis structure of SO2Cl2 contains double bonds between the Sulfur (S) atom & Oxygen (O) atoms and single bonds between the Sulfur (S) atom and Chlorine (Cl) atoms. The Sulfur atom (S) is at the center and it is surrounded by 2 Oxygen atoms (O) and 2 Chlorine atoms (Cl). Let's draw and understand this lewis dot structure step by step.

SO2Cl2 lewis structure, molecular geometry, polar or nonpolar
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".
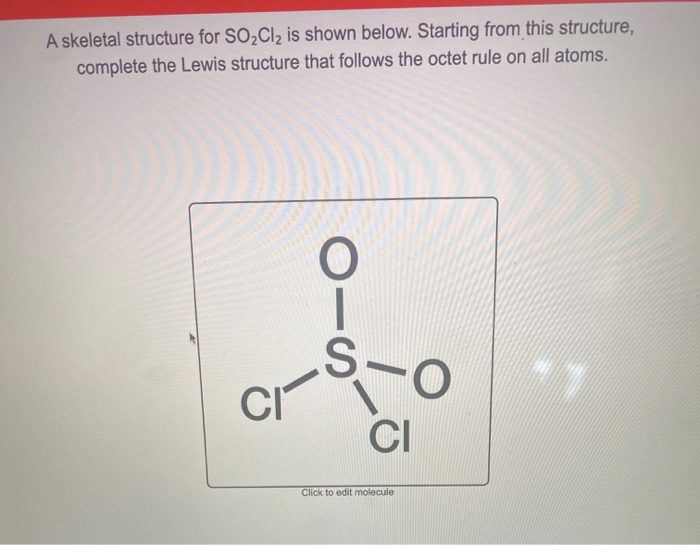
Solved A Skeletal Structure For SO2Cl2 Is Shown Below. St...
To find formal charges in a Lewis structure, for each atom, you should count how many electrons it "owns". Count all of its lone pair electrons, and half of its bonding electrons. The difference between the atom's number of valence electrons and the number it owns is the formal charge. For example, in NH 3, N has 1 lone pair (2 electrons) and 3.
More Lewis Structures Worksheet Answers Ivuyteq
A step-by-step explanation of how to draw the SO2Cl2 Lewis Dot Structure.For the SO2Cl2 structure use the periodic table to find the total number of valence.

SOCl2 Lewis Structure How to Draw the Lewis Structure for SOCl2 YouTube
In socl2 lewis structure we see that as S has larger size and also less electronegative than other atoms it works as central atom in socl2. Sulphur has 6 electrons in the outermost shell out of which 4 electrons participate in sigma bonding with 2 cl atoms and 1 O atom. With O atom S forms 1 sigma and 1 Pi bond.
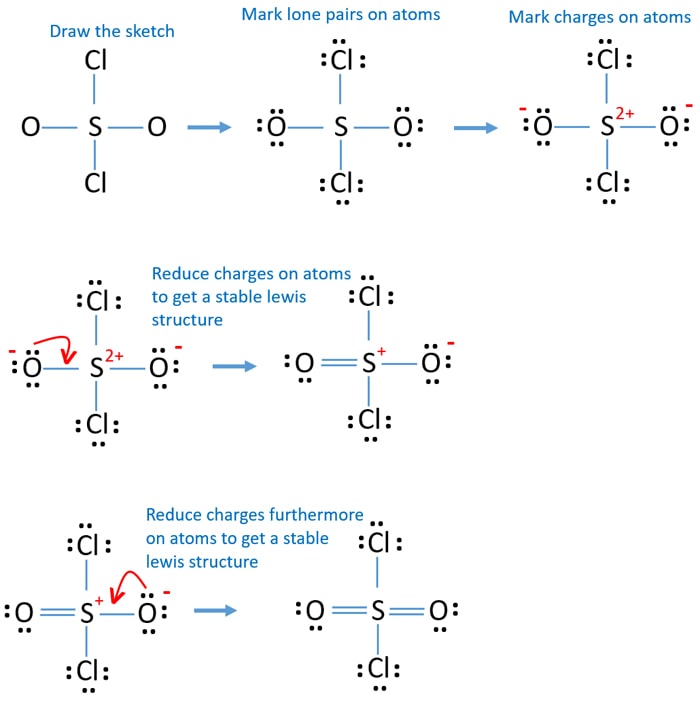
SO2Cl2 (Sulfuryl chloride) Lewis Structure
Sulfuryl chloride (SO 2 Cl 2) contains one sulfur atom, two chlorine atoms and two oxygen atoms. In SO 2 Cl 2 lewis structure, there are two double bonds between sulfur atom and oxygen atoms. Between sulfur and chlorine atoms, there are single bonds. No lone pairs exist on sulfur atom. Lewis structure of SO 2 Cl 2
[Solved] Having trouble with a lewis structure for SO2Cl2 (s central
1 2 3 4 5 6 7 8 9 Share 1.7K views 1 year ago Lewis Structure SO2Cl2 is a chemical formula for Sulfuryl chloride. It is an inorganic compound, and to know more about its properties; we shall.

Lewis dot structure for SO2Cl2 Sulfuryl chloride YouTube
Structure Chemical Safety Laboratory Chemical Safety Summary (LCSS) Datasheet Molecular Formula SO2Cl2 Cl2O2S Synonyms SULFURYL CHLORIDE Sulfuryl dichloride 7791-25-5 Sulfonyl chloride Sulphuryl chloride View More. Molecular Weight 134.97 g/mol Computed by PubChem 2.2 (PubChem release 2021.10.14) Dates Create: 2004-09-16 Modify: 2023-12-30
[Solved] Having trouble with a lewis structure for SO2Cl2 (s central
A step-by-step explanation of how to draw the SOCl2 Lewis Dot Structure (Thionyl chloride).For the SOCl2 structure use the periodic table to find the total n.